The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The southern pine coneworm, Dioryctria amatella (Hulst), also commonly referred to as a pitch moth, is consistently one of the most damaging insect pests of pine seed orchard crops throughout the southeastern United States (Ebel et al. 1980). Less well-recognized is that this widespread insect also attacks other parts of pines (Pinus spp.) besides cones. Caterpillars can be found feeding on and in buds, male and female flowers, shoots, branches and stems of all ages and sizes, as well as in conelets (first-year cones) and second-year cones (Ebel 1965; Ebel et al. 1980; Goolsby et al. 1972).

Credit: R. Scott Cameron, International Paper, www.forestryimages.org
The prevalence of Dioryctria amatella infestations on forest and shade trees pines periodically generates concern over the damage it causes. The most noticeable symptom of an infestation is large masses of pitch exuding from the feeding sites of caterpillars, hence the name "pitch moth." Reddish-brown frass may also be evident at feeding sites and is often mixed with resin. Pitch masses caused by Dioryctria amatella may resemble those of the black turpentine beetle (BTB), Dendroctonus terebrans (Oliv.). However, the coneworm pitch masses are larger, appear irregularly-shaped, and flow for months. BTB pitch masses are typically less than 25 mm (1 in) in diameter, have an obvious entrance hole, solidify in weeks, and are concentrated on the lower bole of large trees (Barnard and Dixon 1983; Goolsby et al. 1972).
In addition to cones, susceptible host material includes: trees under stress, mechanically injured stems or branches, elongating shoots of long leaf (Pinus palustris Mill.) and slash pine (Pinus elliottii Englem.) during the spring, graft and branch unions, conelets infected with the southern cone rust fungus (Cronartium strobilinum (Arth.) Hedge & Hahn) and especially galls caused by the fusiform rust fungus (Cronartium quercuum (Berk.) Miyabe ex Shirai f. sp. fusiforme) (Barnard and Dixon 1983; Ebel et al. 1981; Goolsby et al. 1972). Other than potentially significant losses in seed orchards due to the destruction of flowers, conelets and cones, damage is rarely severe or lethal to trees. The girdling effect of caterpillar feeding, however, can cause dieback of branches, terminals and treetops, and additional weakening of previously damaged sterns (Barnard and Dixon 1983). Feeding injuries also serve as infection courts for the pitch canker fungus, Fusarium subglutinans (Wollenw and Reinking) Nelson, Toussoun and Aarasas (Foltz and Blakeslee 1989).
Distribution
Dioryctria amatella occurs throughout Florida and likely can be found wherever its pine hosts are growing. The natural range of the insect extends across the southeastern US, from Maryland south to Florida and west into Texas (Ebel et al. 1980).
Identification
Dioryctria amatella is one of six species of pine coneworms found in Florida. Others include: the blister coneworm (Dioryctria clarioralis (Walker)), the webbing coneworm (Dioryctria disclusa Heinrich), the south coastal coneworm (Dioryctria ebeli Mutuura & Monroe), the loblolly pine coneworm (Dioryctria merkeli Mutuura & Monroe), and the lesser loblolly pine coneworm (Dioryctria taedivorella Neunzig & Leidy) (Ebel et al. 1980). A seventh species, the bald cypress coneworm (Dioryctria pygmaeella Ragonot), is only known on junipers and cypress. Dioryctria amatella can be distinguished from these other species by distinct adult and larval characteristics and biology, and differences in damage.

Credit: Harry O. Yates III, USDA Forest Service, www.forestryimages.org
Adult moths of Dioryctria amatella have a wingspan of 27 to 32 mm (1 to 1¼ in), with dark grey, to brown, to nearly black forewings boldly patterned with multiple contrasting white patches and zig-zag crossbands. The hindwings are nearly uniformly light grey to tan in color. Larvae range from 1.5 mm (0.06 in) upon hatching to 25 mm (1 in) at maturity. When young, their bodies are nearly white with seven longitudinal stripes and a brown head. Older larvae are dark reddish to purplish brown above and whitish green on the underside. Abdominal segments exhibit beadlike patterns of small black pits and dark elevated setal bases (Ebel et al. 1980).

Credit: Larry R. Barbar, USDA Forest Service, www.forestryimages.org
Biology
In Florida, the southern pine coneworm produces one to four generations per year, depending on whether larval diapause occurs during the spring, early summer, or at all. The overlap of life stages among generations yields varying degrees of adult moth activity from early April through early November in northern Florida (Merkel and Fatzinger 1971). The insect overwinters predominantly as early instar larvae, at the base of persistent cones, under bud scales and in fusiform galls on branches and sterns. As larvae become active in January, they may continue to feed in overwintering sites or migrate to feed on developing male and female flowers and vegetative buds. Following flower and bud feeding, larvae usually migrate a second time, infesting expanding shoots or young second-year cones during early spring. Once in shoots, larvae may undergo diapause, complete their development, or migrate again to young second-year cones. When larvae eventually pupate, Dioryctria amatella is the only pine coneworm in the southern US that does so within infested material (Ebel 1965).
In April and May, the next generation of caterpillars readily infests conelets infected with southern cone rust, as well as healthy second-year cones. These larvae, as well those of subsequent generations, also may migrate from infested to uninfested cones before pupating. Later generations continue to infest second-year cones from summer through fall (Ebel 1965). This relatively complex cycle is depicted in Figure 4.
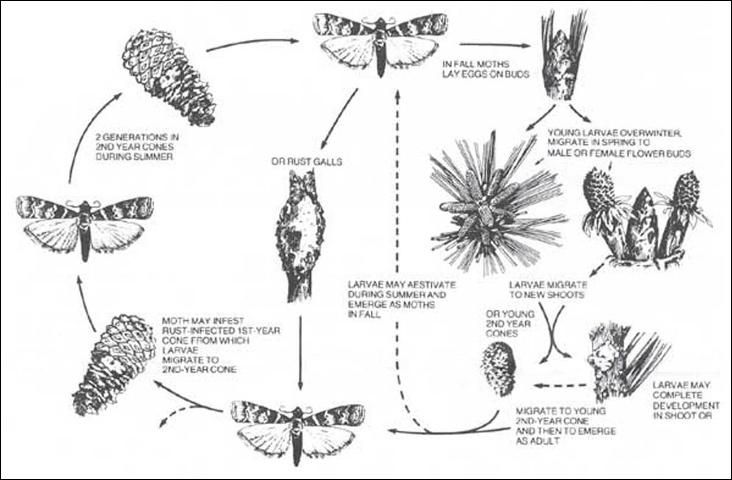
Credit: Hedlin et al. (1981)
Host Plants
All species of Pinus native to Florida are utilized as hosts by D. amatella. This list includes loblolly (Pinus taeda L.), longleaf, pond (Pinus serotina Michx.), sand (Pinus clausa (Chapm. ex Englem.)), shortleaf (Pinus echinata Mill.), slash (Pinus elliottii Englem.), and spruce (Pinus glabra Walt.) pines. Virginia pine, Pinus virginiana Mill., which is sometimes ill advisedly grown in Florida for Christmas trees, is often infested by Dioryctria amatella. Eastern white pine, Pinus strobus L., is the only Pinus spp. in Florida that is not a suitable host (Ebel et al. 1980). Within slash pine, and probably other host species, individual trees exhibit pronounced differences in their inherent degree of susceptibility or resistance to Dioryctria amatella infestations (Merkel et al. 1965).
Damage
In cones, larval feeding causes damage ranging from evident tunnels to wholly excavated cavities, resulting in partial to complete seed loss of infested individuals (Ebel et al. 1980). Other forms of damage are pictured, in part, in images below. Foltz and Blakeslee (1989) found that the abundance of Dioryctria amatella infestations in young, experimental slash pine plantations increased with the intensity of applied cultural practices (e.g., fertilization, competition control and irrigation). In the late 1990s, a four-year-old longleaf plantation on a scalped agricultural field in Lafayette County exhibited an estimated 5% terminal dieback due to shoot infestations. The unknown ramifications of trends towards more intensive, high-yield management of many loblolly, slash and longleaf plantations may alter the pest status and management considerations for Dioryctria amatella in the future.

Credit: Bernard H. Ebel, USDA Forest Service, www.forestryimages.org

Credit: Larry R. Barber, USDA Forest Service, www.forestryimages.org

Credit: Harry O. Yates III, USDA Forest Service, www.forestryimages.org

Credit: Bernard H. Ebel, USDA Forest Service, www.forestryimages.org

Credit: Andrew J. Boone, South Carolina Forestry Commission, www.forestryimages.org
Management
Various insecticides are currently registered for and routinely used in intensively managed pine seed orchards to successfully prevent and/or minimize seed losses due to coneworms. The presence and timing of Dioryctria amatella and most other coneworms can be determined by using commercially available pheromone lures and traps. In most conventional forestry and shade tree settings, there is little practical potential for insecticide use due to the random nature of attacks, the inability of most insecticides to control existing infestations, the relatively limited impact of damage, and the high cost-benefit ratio.
Recommended management strategies include promotion and maintenance of tree health and removal and destruction of seriously infested and/or rust infected stems and branches. Mechanical injuries to branches and stems should be avoided because Dioryctria amatella is attracted to volatiles that emanate from tree wounds (Hanula et al. 1985). In Florida, eight species of Hymenoptera (three braconids, three ichneumonids and two eulophids) and two species of tachinid flies have been reported as natural enemies of Dioryctria amatella larvae or pupae. It appears, however, that the combined effects of these parasitoids are not enough to substantially reduce or control populations of this coneworm (Belmont and Habeck 1983; Ebel 1965).
Selected References
Barnard E.L., W.N. Dixon. 1993. Insects and diseases: Important problems of Florida's forest and shade tree resources. Florida Department of Agriculture & Consumer Services, Division of Forestry. Bulletin No. 196-A. 120 p.
Belmont R.A., D.H. Habeck. 1983. Parasitoids of Dioryctria spp. (Pyralidae: Lepidoptera) coneworms in slash pine seed production areas of north Florida. Florida Entomologist 66: 399–407.
Ebel, BH. 1965. The Dioryctria coneworms of North Florida pines (Lepidoptera: Phycitidae). Annals of the Entomological Society of America 58: 623–630.
Ebel B.H., T.H. Flavell, L.E. Drake, H.O. Yates III, G.L. DeBarr. 1980. Seed and cone insects of southern pines. U.S. Department of Agriculture, Forest Service, Southeastern Forest Experiment Station and Southeastern Area State and Private Forestry. General Technical Report SE-8. 43 p.
Foltz J.L., G.M. Blakeslee. 1989. Insects associated with the intensive culture of Pinus ellottii and P. taeda in Florida. pp. 19–26. In R.I. Alfaro, S.G. Glover. (eds.). Insects affecting reforestation: Biology and damage. Forestry Canada. Victoria, British Columbia, Canada.
Goolsby R.P., J.L. Ruehle, H.O. Yates III. 1972. Insects and diseases of seed orchards in the South. Georgia Forest Research Council. Report No. 28. 25 p.
Hanula J.L., C.W. Berisford, G.L. DeBarr. 1985. Monoterpene oviposition stimulants of Dioryctria amatella in volatiles from fusiform rust galls and second-year loblolly pine cones. Journal of Chemical Ecology 11: 943–952.
Hedlin A.F., H.O. Yates III, E. Cibrián Tovar, B.H. Ebel, T.W. Koerber, E.P. Merkel. 1981. Cone and seed insects of North American conifers. Canadian Forestry Service, U.S. Forest Service, Secretaria de Agricultura y Recursos Hidráulicos, Mexico. 122 p.
Merkel E.P., C.W. Fatzinger. 1971. Periodic abundance of pine cone-infesting Lepidoptera in black light traps and sleeve cages in North Florida. Florida Entomologist 54: 53–61.
Merkel E.P., A.E. Squillace, G.W. Bangston. 1965. Evidence of inherent resistance to Dioryctria infestation in slash pine. pp. 96–99. In (eds) Proceedings of the Eighth Annual Southern Forest Tree Improvement Conference, Savannah, Georgia.