The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
The seagrape borer, Hexeris enhydris Grote, is a species of moth that is native to Florida and the Caribbean Region whose larvae bore into twigs of seagrape, Coccoloba uvifera L., and a closely related species, pigeon-plum, Coccoloba diversifolia Jacquin (Polygonaceae). Seagrape is a native shrub or small tree and pigeon-plum is a moderately large tree at maturity. Both grow naturally in coastal habitats in Florida and the Caribbean Region. Seagrape, and to a lesser extent pigeon-plum, are also grown in urban landscapes in southern Florida and in many Caribbean countries.

Credit: F. W. Howard, UF/IFAS

Credit: F. W. Howard, UF/IFAS

Credit: F. W. Howard, UF/IFAS
Distribution
The seagrape borer is found throughout the range of seagrape in Florida, i.e., in coastal areas of central and southern Florida and the Keys (Chellman 1978). It is also found in Cuba (Grote 1875). It is probably more widely distributed in Tropical America than has been reported, because seagrape itself is present on coastal sites throughout the Caribbean Region, and the genus Coccoloba contains about 180 species distributed in Tropical America, some of which may be unreported hosts.
Description
Adult
The adult moth is yellowish to pale brown with rusty wavy lines on the forewings and with a wingspan of 34 to 38 mm (1 1/3–1 ½ in). The prominent labial palps extend forward twice the length of the head.

Credit: F. W. Howard, UF/IFAS
Larva
The larva is apodous (legless), which is a trait typical of wood-boring insect larvae. The mature larva is about 15 mm (~2/3 in) in length.

Credit: F. W. Howard, UF/IFAS
Pupa
The pupa is brown and about the same length as the mature larva (Solomon 1995).
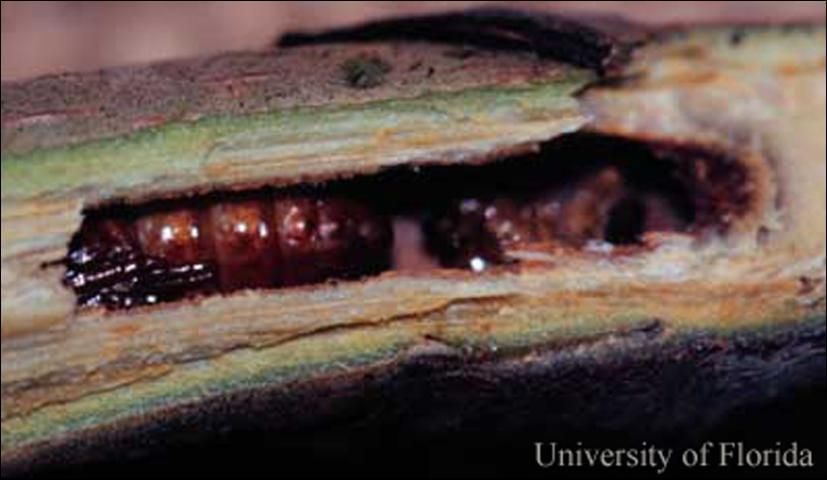
Credit: F. W. Howard, UF/IFAS
Biology
The adult moths have been observed flying during both the warmer and cooler months in Florida (Chellman 1978). The females oviposit on the surfaces of twigs and leaves, as Lepidoptera do not have ovipositors that can pierce plant tissue for insertion of eggs. Upon hatching, the larva feeds externally for a brief period before it begins boring into plant tissue. It bores into the twig or leaf petiole, or more rarely into the leaf midvein. They sometimes cut small holes in the walls of the tunnel to the outside, through which frass is expelled (Solomon 1995).
The duration of larval development is not known. Pupation takes place inside the tunnel. Whether the insect prefers seagrape or pigeon-plum is not known. The borers have been observed and collected more often from seagrape than from pigeon-plum, since the latter is far less common as an ornamental plant. Adult moths emerging from the pupal case find their way to an exit, leave the tunnel, and fly to find mates. Lepidoptera are generally nectar feeders, but the food of adult seagrape borer moths has not been determined.
Damage
Twigs that have been hollowed out by seagrape borers are easy to detect, especially if, as often happens, their damage has resulted in necrosis of the leaves attached to the twig. Leaves of seagrape are large, typically 16 to 20 cm (6 ¼-7 ¾ in) long and wide, and thus the dead leaves, which turn yellow to reddish (sometimes referred to as "flags") can be spotted from a distance. Twigs containing borers usually have necrotic areas and holes with frass spilling out. Splitting the twig may reveal the larva or pupa. If the insect has completed its development, an empty pupal case is present. Older damage often consists of dead hollow twigs that are frayed at the terminal (Johnson and Lyon 1991; Solomon 1995).

Credit: F. W. Howard, UF/IFAS

Credit: F. W. Howard, UF/IFAS
Management
The seagrape borer is attacked by at least one unidentified species of hymenopterous parasitoid (Moon and Stiling 2004). Natural enemies and other factors likely keep the borer at tolerable population levels. Although it is widely distributed on seagrape trees, usually only a very small percentage of the twigs of seagrape trees are attacked by the seagrape borer, and the damage, albeit conspicuous to those familiar with it, is not noticeable to most people. It is only in unusual cases that the borer population may be sufficient to cause extensive dieback of twigs. Damage by this borer may be more serious on small trees in nurseries. Pruning and destroying twigs infested with the borers has been recommended as a method of reducing populations (Chellman 1978), but the effectiveness of this method has not been evaluated. Control with insecticides has likewise not been investigated in recent years.
Acknowledgements
The author thanks Dr. Robert Pemberton of the USDA-ARS, Invasive Plants Laboratory, Fort Lauderdale; and Dr. William Kern, UF/IFAS Fort Lauderdale Research and Education Center, for reviewing the manuscript.
Selected References
Chellman CW. 1978. Pests and problems of south Florida trees and palms. Florida Department of Agriculture and Consumer Services, Division of Forestry, Tallahassee, Florida.
Grote AR. 1875. On certain species of moths from Florida. Canadian Entomologist 7: 173–176.
Johnson WT, Lyon HH. 1991. Insects that feed on trees and shrubs. Second, revised ed. Comstock Publishing Associates, Cornell University Press, Ithaca and London.
Moon DC, Stiling P. 2004. The influence of a salinity and nutrient gradient on coastal vs. upland tritrophic complexes. Ecology 85: 2709–2716.
Solomon JD. 1995. Guide to insect borers of North American broadleaf trees and shrubs. U.S. Forest Service, U.S. Department of Agriculture, Washington, D.C.