Introduction
The black turpentine beetle, Dendroctonus terebrans (Olivier), or BTB, is one of five common species of pine bark beetles in the southeastern United States. Black turpentine beetles bore into the inner bark of stressed or injured pines (Pinus spp.), where they breed and feed on phloem tissue. Adults are strongly attracted to volatile pine odors and readily breed in fresh stumps. Attacks on standing trees usually occur on the lower 1 to 2 m of the trunk or on large roots. Light attacks may kill only localized sections of phloem tissue, but numerous attacks per stem result in tree mortality. Infestations commonly occur in pine stands affected by recent logging activity (e.g., thinning), fire, mechanical injury, storm damage, climatic stress, or competition (USDA Forest Service 1985; Dixon 1986).
Historically, black turpentine beetle has been a major pest of pines wounded or treated with herbicides in naval stores production (Smith and Lee 1972; Goldman et al. 1979). When local populations are sufficiently high, apparently healthy trees can be killed (Smith and Lee 1972). During the 1950s, black turpentine beetle damaged 37 million board feet of timber and contributed to the financial collapse of turpentine farms (Merkel 1981).
In typical forests, black turpentine beetle infestations do not exhibit the rapid and devastating expansion characteristic of the closely related southern pine beetle, Dendroctonus frontalis, and typically result in scattered, patchy and limited pine mortality. However, in stands where stress conditions are frequent or persistent, black turpentine beetle can become a chronic pest and cause significant mortality over an extended period of time.
Distribution
The black turpentine beetle is found throughout the eastern United States from coastal New Hampshire south through Florida and west to Texas and Missouri. It is an interesting distribution since the black turpentine beetle occupies the part of the continent not occupied by the much more widespread red turpentine beetle (Dendroctonus valens LeConte). The red turpentine beetle is very similar in appearance and behavior, and occurs from Alaska to Mexico and eastward to New England, but does not occur in the southeastern US. The two species co-occur only in a narrow zone where their ranges overlap.
Description
Adults are cylindrical, dark reddish-brown to black, and 5 to 8 mm in length. This is the largest bark beetle species in the southeastern US. The head is convex in front and has clubbed antennae. The pronotum (the hard cover on top of the insect's midsection) is much narrower in the front than in the rear.
The only other Dendroctonus species in Florida is Dendroctonus frontalis Zimmermann, the southern pine beetle. It is similarly shaped but much smaller than the black turpentine beetle, being just 2 to 3 mm in length.
Two other genera commonly found in pines are Ips and Orthotomicus. Species in these genera are readily distinguished from Dendroctonus beetles by (1) the heads being concealed beneath the pronotum rather than being exposed, and (2) the posterior of the abdomen being flattened or impressed rather than being rounded.

Credit: Adam Black and Jiri Hulcr, UF/IFAS

Credit: David Almquist, UF/IFAS
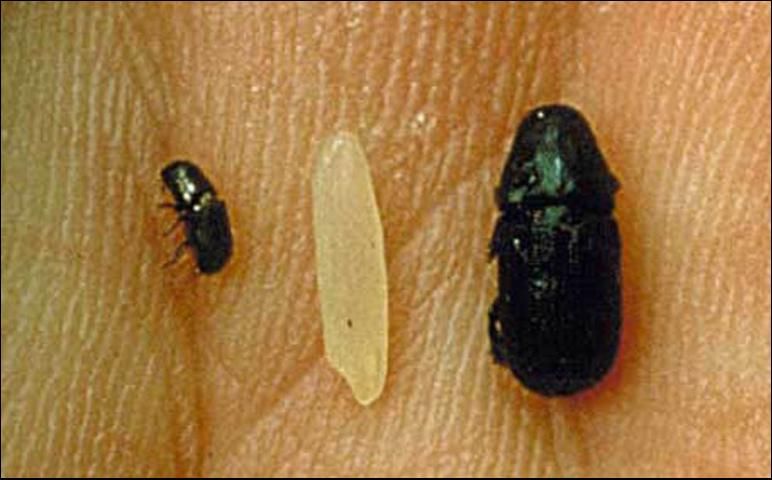
Credit: Southern Forest Insect Work Conference Archives, www.forestryimages.org
Mature larvae are about 12 mm long, creamy white, legless, and have reddish-brown heads. Pupae are waxy-white and of size similar to adults (Goyer et al. 1980; USDA Forest Service 1985).
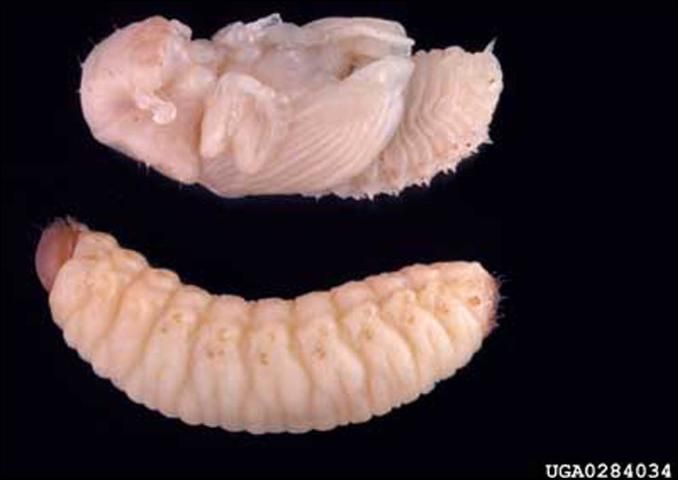
Credit: Gerald J. Lenhard, www.forestryimages.org
Biology
Adult females initiate attack on the lower tree bole or root collar by boring a hole through the outer bark. Trees with sufficient resin pressure typically produce a large mass of resin that combines with reddish boring dust to form a pitch tube at the site of attack. A female beetle that successfully overcomes this resinous host defense mechanism initiates gallery construction in the phloem and is then joined by a male.
Tree colonization is mediated through beetle pheromones and volatile host attractants (Smith et al. 1993). The black turpentine beetle can be attracted (and thus monitored) using turpentine, a complex chemical mixture dominated by alpha-pinene and beta-pinene. However, it does not respond to some of the currently used simple terpene-based lures such as a mixture of alpha- and beta-pinene without the remaining trace volatiles (Hulcr and Wakarchuk unpublished data).

Credit: Wayne Dixon, FDACS-DPI, www.forestryimages.org

Credit: Albert E. Mayfield III, FDACS
The female spends about two weeks constructing a gallery about 1 cm wide and 20 to 30 cm long on the face of the sapwood, typically in a downward direction from the entrance hole. The gallery frequently extends below the ground surface. The female then lays about 100 eggs in an elongate batch along one side of the gallery. Eggs hatch 10 to 14 days later.

Credit: Ronald F. Billings, Texas Forest Service; www.forestryimages.org
Larvae feed side by side, creating a large, fan-shaped gallery or cavity that may fill with resin and frass. Mature larvae eventually tunnel away from the expanded cavity and create pupation cells. The communal feeding of all black turpentine beetle larvae is a unique feature among bark beetles, and can be used to identify a black turpentine beetle gallery. In most species, each larva typically bores its own tunnel.

Credit: Jiri Hulcr, UF/IFAS
Young adults chew holes in the bark, exit their brood tree and disperse to colonize suitable host material. There are up to three overlapping generations per year in Florida and all life stages can be found year round (Smith and Lee 1972; Godbee and Franklin 1976; USDA Forest Service 1985). Fatzinger (1985) found peak trap captures in North Florida to occur in March, July and October.
The black turpentine beetle is capable of vectoring blue stain fungi into the sapwood of its hosts (Barras and Perry 1971; Rane and Tattar 1987). Because black turpentine beetle-attacked trees often survive, however, fungal colonization of the xylem may be less frequent, less pathogenic, or less extensive than blue stain introductions made by Dendroctonus frontalis and Ips engravers that result in rapid plugging of the xylem and hastened tree mortality. Tree mortality from black turpentine beetle is, therefore, often a result of accumulated attacks over multiple generations or years.
Hosts
All species of southern pines (Pinus spp.) and red spruce (Picea rubens Sarg.) are suitable hosts. Slash (Pinus elliottii Engelm.) and loblolly (Pinus taeda L.) pines are common hosts in the southeastern US (USDA Forest Service 1985). In other locations, native and non-native hosts include pitch pine (Pinus rigida Mill.), eastern white pine (Pinus strobus L.), black pine (Pinus thunbergiana Franco) and Scots pine (Pinus sylvestris L.) (Wood 1982; Rane and Tattar 1987). Black turpentine beetle can cause significant mortality on some non-native ornamental species, such as the Japanese black pine, which ceased to be planted in New England in the 1990s (Staeben et al. 2010). This impact on exotic pine species raises concerns about the potential of this species to become an invasive pest overseas.
Detection
Signs and symptoms of black turpentine beetle attack include:
-
large (2 to 5 cm wide) pinkish-white, reddish-brown, or purplish-gray pitch tubes on the lower bole of the tree, which may exhibit an entrance hole 3 to 5 mm wide (Figure 5),
-
reddish-brown boring dust,
-
accumulations of crystallized pitch pellets on the ground at the base of the tree,
-
large vertical or fan-shaped galleries in the inner bark (Figure 7), and
-
the entire tree crown gradually fading from green to yellow to red to brown.
Note: Pitch tubes in which the resin has dried without any reddish sawdust indicate failed attacks.
Prevention and Management
Promoting and maintaining the health of pine trees and stands is the best way to prevent black turpentine beetle attacks.
Preventative strategies in forest stands include:
-
planting species that are appropriate to the site,
-
thinning overstocked stands and maintaining an average basal area of less than 80 sq. ft. per acre,
-
conducting prescribed burns and other treatments to control competing vegetation without damaging residual trees,
-
harvesting damaged, declining, or recently-dead trees,
-
conducting harvest operations during the cooler fall and winter months,
-
avoiding root and bole damage to residual trees during management operations,
-
keeping stump heights as low as possible, and
-
keeping piles of logging slash and stacks of fresh pine firewood away from the base of live pines.
Thinning should be conducted before trees begin declining in growth due to root and crown competition. Avoid thinning when active bark beetle infestations are present within or adjacent to the stand. In areas where annosum root disease, caused by the fungus Heterobasidion annosum (Fr.:Fr.) Bref., has been or could be a problem, consider treating fresh-cut stump surfaces with borax following cool-season thinnings to help prevent root disease development in residual pines (Barnard 1999).
As for control, when black turpentine beetle infestations are small and/or sparsely scattered throughout a stand, the best course of action is usually to let them die out on their own. Cutting and removing isolated infested trees or small "spot" infestations, as is done to control southern pine beetle infestations, is not recommended. In some cases such selective removals may exacerbate black turpentine beetle problems by producing fresh host odors, fresh breeding material, and additional stress or injury to the residual stand. If tree mortality is progressing to unacceptable levels, a stand-level clearcut or a block removal of a generally infested area is preferable to selection harvests. Use of traps to control infestations and prevent attacks has not been successful (Fatzinger 1985).
For urban and residential landscape pines, preventative strategies include:
-
avoiding compaction of, physical damage to, or pavement over the root zones of pines,
-
avoiding trunk wounds,
-
providing adequate spacing (4–6 m) between trees,
-
minimizing competing vegetation beneath pines,
-
maintaining proper soil nutrient status through an acidic needle or pine bark mulch over the root zone, and by not routinely watering turf grasses beneath pines, and
-
providing supplemental deep watering during extended drought periods.
In some cases, the application of an approved insecticide to the lower 2 to 3 m of trunk may be warranted to protect high-value landscape trees from infestation. As a rule of thumb, trees on which the number of black turpentine beetle pitch tubes is less than the diameter of the tree in inches are more likely to survive than those with greater attack densities; such trees may be good candidates for insecticidal treatment to prevent additional attacks.
Contact your local UF/IFAS Extension office for current insecticide recommendations. Infested trees should be removed carefully to avoid injury to surrounding pines.
Selected References
Barnard EL. 1999. Annosum Root Rot of Pines in Florida. Plant Pathology Circular No. 398, Florida Department of Agriculture and Consumer Services, Division of Plant Industry, Gainesville. 5 p.
Barras SJ, Perry T. 1971. Leptographium terebrantis sp. nov. associated with Dendroctonus terebrans in loblolly pine. Mycopathologia et Mycologia applicata 43:1–10.
Dixon WN. 1986. Black turpentine beetle. Florida Department of Agriculture and Consumer Services, Division of Forestry, Gainesville. Forest and Shade Treet Pest Leaflet No. 4.
Fatzinger CF. 1985. Turpentine-baited traps capture black turpentine beetles and other forest Coleoptera but do not prevent attacks on pines. pp. 26–31. In Branham SJ, Thatcher RC. (eds.). Integrated pest management symposium: the proceedings. April 15–18, 1985. Asheville, NC. USDA Forest Service, Southern Forest Experiment Station. General Technical Bulletin SO-56.
Godbee JF, Franklin RT. 1976. Attraction, attack patterns and seasonal activity of the black turpentine beetle. Annals of the Entomological Society of America 69: 653–655.
Goldman SE, Cleveland GD, Parker JA. 1979. Lightwood induction and associated beetle attacks on slash pine. Forest Science 25: 80–83.
Goyer RA, Lenhard GJ, Nebeker TE, Jarrard LD. 1980. How to identify common insect associates of the southern pine beetle. Southern Pine Beetle Handbook. United States Department of Agriculture, Combined Forest Pest Research and Development Program. Agriculture Handbook No. 563.
Merkel EP. 1981. Control of the black turpentine beetle. Georgia Forest Research Paper No. 15.
Rane KK, Tattar TA. 1987. Pathogenicity of blue-stain fungi associated with Dendroctonus terebrans. Plant Disease 71: 879–883.
Smith MT, Salom SM, Payne TL. 1993. The southern pine bark beetle guild: an historical review of the research on the semiochemical-based communication system of the five principle species. Virginia Agricultural Experiment Station, Bulletin No. 93-4. Blacksburg, VA. 106 p.
Smith RH, Lee, III RE. (1972). Black turpentine beetle. USDA Forest Service. Forest Pest Leaflet 12. (30 March 2015).
Staeben J, Clarke S, Gandhi K. 2010. Forest insect and disease leaflet 12: Black turpentine beetle. USDA Forest Service, Pacific Northwest Region (R6), Portland, Oregon.
USDA Forest Service. 1985. Insects of Eastern Forests. Misc. Publication No. 1426. Washington, DC. 608 p.
Wood SL. 1982. The bark and ambrosia beetles of North and Central America (Coleoptera: Scolytidae), a taxonomic monograph. Great Basin Naturalist Memoirs. No. 6. 1359 p.