Introduction
The cassius blue, Leptotes cassius (Cramer), is a beautiful tiny butterfly that is locally common throughout peninsular Florida—particularly along the coasts. It is sometimes known as the tropical striped blue (Scott 1986). There are two subspecies in the United States, theonus in Florida and striata in Texas. Blues in the genus Leptotes are collectively known as the "zebra blues" (Brower 2008) because of the characteristic dark stripes on the undersurface of the wings.
Distribution
The cassius blue, Leptotes cassius theonus, is resident in southern peninsular Florida but occasionally strays to more northern areas. It is cold sensitive and cannot survive even the winters of northern Florida. The mechanism by which individuals arrive at more northern localities is not known. Perhaps some are carried by wind. Daniels (2005) found larvae on ornamental leadwort plants in South Carolina that had been shipped from Florida and suggested that piggybacking on ornamental host plants may account for the some of the northern strays.
Description
Adults: The wing spread is 3/4–15/16 inches (20–33 mm) (Opler and Malikul 1992). The undersides of the wings are striped with two eyespots on the margin of each hind wing. Males are pale to bright blue above. Females are bluish-white to white above on basal areas of wings with broad dark borders on the front wings and a dark spot on the rear margin of the hindwing (Cech and Tudor 2005; Minno and Minno 1999).

Credit: Jerry Butler, UF/IFAS

Credit: Jerry Butler, UF/IFAS
Eggs: The eggs are whitish-green and the surface is sculptured with a network of knobs and ridges.
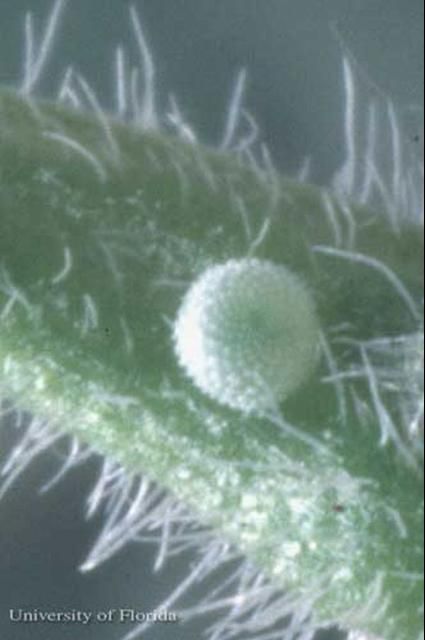
Credit: Jerry Butler, UF/IFAS
Larvae: Ful-grown larvae are approximately 0.5 inch in length (Minno et al. 2005). Body color is green with faint dark markings or patterned with faint white markings (formed by numerous tiny white hair-bearing tubercles [chalazae]) or red markings and white chevrons.

Credit: Jerry Butler, UF/IFAS
Pupae: The pupae are tan with dark markings and numerous short white hairs.

Credit: Jerry Butler, UF/IFAS
Life Cycle and Biology
The cassius blue is found throughout the year in Florida and has at least three generations per year (Glassberg et al.; Minno et al. 2005).
The eggs are laid singly on the flower buds of the host plants, and larvae eat the buds, flowers, and developing seeds (Minno and Minno 1999; Minno et al. 2005). There are four larval instars in Florida (Downey and Allyn 1979).
The larvae are well-camouflaged and may be attended by ants for which they provide sugary secretions from a honey gland on the dorsal surface of the seventh abdominal segment (Downey and Allyn 1979; Opler et al. 2009).
As is the case with most lycaenids, pupae stridulate with a scraper and file in the dorsal cleft between the fifth and sixth abdominal segments (Downey and Allyn 1979). The stridulation is assumed to serve a defensive purpose.
Downey and Allyn (1979) found a braconid wasp in the genus Pelecystoma parasitizing larvae, and the tachinid fly Eusisyropa boarmiae [Coq.] parasitizing pupae. In a collection of eggs from early June, they found 17% parasitized by the generalist egg parasite Trichogramma minutum Riley.
Adults feed on nectar from a wide variety of flowers.
Hosts
The larval hosts of the cassius blue are a variety of vines, shrubs, and trees in the pea family (Fabaceae) (Minno and Emmel 1993; Minno and Minno 1999; Minno et al. 2005) and leadworts (Plumbaginaceae) including:
-
Milkpeas, Galactia spp. (Fabaceae) (Figures 6 & 7)
![Figure 6 Figure 6. Downy milkpea, Galactia volubilis [L.] Britton, (Fabaceae) a host of the cassius blue, Leptotes cassius (Cramer).](/image/IN829/697681/14035371/14035371-2048.webp)
Credit: Don Hall, UF/IFAS
![Figure 7 Figure 7. Florida hammock milkpea, Galactia striata [Jacq.] Urb., (Fabaceae) a host of the cassius blue, Leptotes cassius (Cramer).](/image/IN829/697681/2257369/2257369-2048.webp)
Credit: Don Hall, UF/IFAS
-
Blackbeads, Pithecellobium spp., (Fabaceae) (Figures 8 and 9)

Credit: Don Hall, UF/IFAS
![Figure 9 Figure 9. Catclaw blackbead, Pithecellobium unguis-cati [L.] Mart., (Fabaceae) a host of the cassius blue, Leptotes cassius (Cramer).](/image/IN829/697681/15222092/15222092-2048.webp)
Credit: Don Hall, UF/IFAS
-
Hairy cowpea, Vigna luteola [Jacq.] Benth., (Fabaceae)
-
False tamarind, Lysiloma latisiliquum [L.] Benth., (Fabaceae) (Figure 10)
![Figure 10 Figure 10. False tamarind, Lysiloma latisiliquum [L.]Benth. (Fabaceae), a host of the cassius blue, Leptotes cassius (Cramer).](/image/IN829/697681/15241511/15241511-2048.webp)
Credit: Don Hall, UF/IFAS
-
Florida fishpoison tree or Jamaican dogwood, Piscidia piscipula [L.] Sarg. (Fabaceae)
-
Rosarypea, Abrus precatorius L., (Fabaceae) (also known by many other common names) (Figure 11). This species is invasive and disruptive to native plant communities (UF/IFAS Assessment 2019), and its use in Florida is prohibited. Also, its seed is extremely poisonous (INCHEM undated). Therefore, it should never be planted.

Credit: Don Hall, UF/IFAS
-
Cape leadwort, Plumbago auriculata Lam. (Figure 12), a widely planted exotic ornamental in the leadwort family (Plumbaginaceae).

Credit: Don Hall, UF/IFAS
-
Doctorbush, Plumbago zeylanica L., (Plumbaginaceae), a native leadwort species, is also used (Minno and Minno 1999).
For photographs of Vigna luteola, Piscidia piscipula, and Plumbago zeylanica, see their respective species pages at Wunderlin et al. (2019).
Selected References
Brower A. V. Z. (May 2008). Leptotes: the zebra blues. Tree of Life web project. (31 October 2019)
Daniels J. C. 2005. "Piggybacking northward: movement of Leptotes cassius (Lycaenidae: Lycaeninae) throughout the Southeast." Journal of the Lepidopterists' Society 59: 234.
Downey J. C., and A. C. Allyn. 1979. "Morphology and biology of the immature stages of Leptotes cassius theonus (Lepidoptera: Lycaenidae)." Bulletin of the Allyn Museum 55: 1–27.
UF/IFAS Assessment. 2019. Abrus precatorius. (https://assessment.ifas.ufl.edu/assessments/abrus-precatorius/)
Glassberg J., C. Minno, and J. V. Calhoun. 2000. Butterflies through Binoculars: Florida. New York, NY: Oxford University Press.
INCHEM. Abrus precatorius L. International Programme on Chemical Safety. (https://inchem.org/documents/pims/plant/abruspre.htm) (31 October 2019)
Miller J. Y. (editor). 1992. The Common Names of North American Butterflies. Washington, DC: Smithsonian Institution Press.
Minno M. C., J. F. Butler, and D. W. Hall. 2005. Florida Butterfly Caterpillars and their Host Plants. Gainesville, FL: University Press of Florida.
Minno M. C., and T. C. Emmel. 1993. Butterflies of the Florida Keys. Gainesville, FL: Scientific Publishers.
Minno M. C., and M. Minno. 1999. Florida Butterfly Gardening: A Complete Guide to Attracting, Identifying, and Enjoying Butterflies. Gainesville, FL: University Press of Florida.
Opler, P. A., K. Lott, and T. Naberhaus. (2009). Butterflies and Moths of North America. (2 August 2013)
Opler P. A., and V. Malikul. 1992. A Field Guide to Eastern Butterflies (Peterson Field Guide Series). New York, NY: Houghton Mifflin Company.
Scott J. A. 1986. The Butterflies of North America: A Natural History and Field Guide. Stanford, CA: Stanford University Press.
Wunderlin R. P., and B. F. Hansen. 2003. Guide to the Vascular Plants of Florida. 2nd ed. Gainesville, FL: University Press of Florida.
Wunderlin R. P., B. F. Hansen, A. R. Franck, and F. B. Essig. 2019. Atlas of Florida Plants. (https://florida.plantatlas.usf.edu) Institute for Systematic Botany. Tampa, FL: University of South Florida.