Introduction
The boisduval scale, Diaspis boisduvalii Signoret, is an economically important pest of orchids and was reported as the most important insect pest of orchids in Florida (Dekle 1965). Miller and Davidson (2005) list boisduval scale as one of the 43 most serious worldwide armored scale pests.
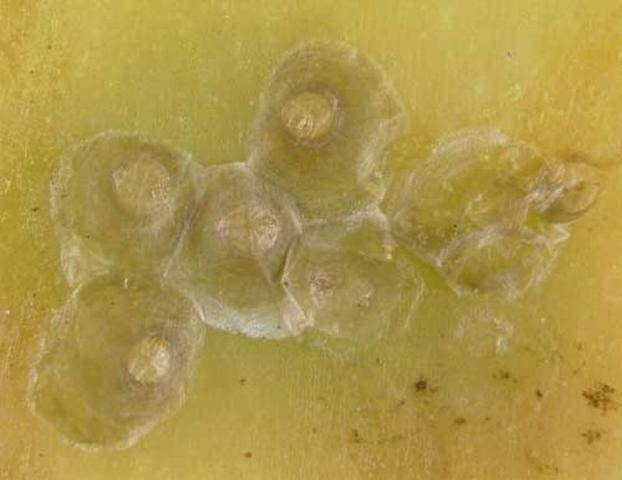
Credit: Lyle J. Buss, UF/IFAS
Distribution
The boisduval scale occurs throughout the tropics and as a greenhouse pest in more temperate climates (Dekle 1965, Miller and Davidson 2005).
Description
Females are white to light yellow, approximately 0.05–0.09 inches (1.2–2.25 mm) in diameter, circular or oval in shape, and covered with a centrally located, white-transparent, flat circular or oval shed skin. When the scale cover is removed, a single, horn-like projection on either side of the body, near the head and thorax may be visible (Dekle 1965, Howard et al. 2001, Miller and Davidson 2005).
Males are oval to elongate in shape, with a white cover and marginal shed skin. Males measure approximately 0.04 inches (1mm) in length.
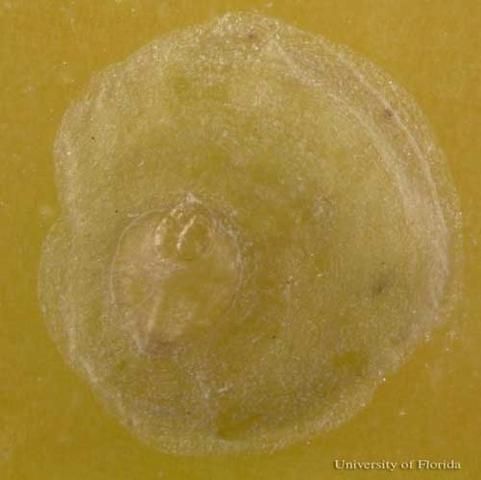
Credit: Lyle, J. Buss, UF/IFAS
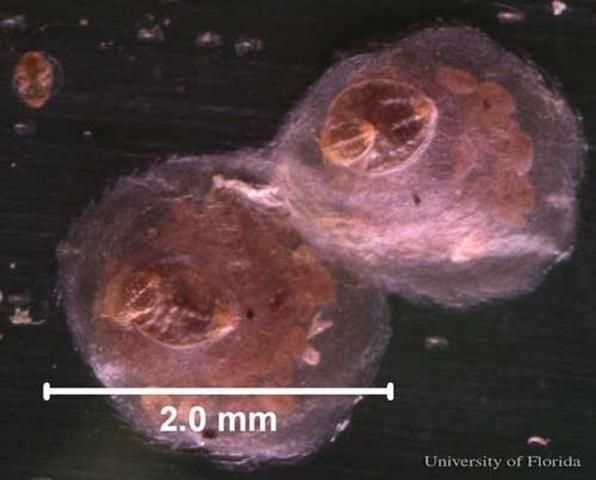
Credit: UF/IFAS

Credit: Avas Hamon, Florida Department of Agriculture and Consumer Services, Division of Plant Industry

Credit: Heidi M. Bowman, UF/IFAS
Life Cycle
Each adult female may produce up to 200 eggs and live as long as seven months. Eggs hatch within five to seven days and become crawlers. Egg color depends upon maturity, and ranges from clear to yellow, and finally orange. The development from egg to the adult stage averages 33 days for males and 50 days for females (Miller and Davidson 2005).

Credit: Heidi M. Bowman, UF/IFAS
Hosts
This species has been recorded on over 15 families and 65 genera of plants in Florida alone, but is most commonly found on orchids and palms.
Within the palm hosts (family Arecaceae) the following genera are susceptible (Howard et al. 2001, Miller and Davidson 2005):
-
Acoelorraphe
-
Acrocomia
-
Archontophoenix
-
Areca
-
Bactris
-
Butia—pindo palm
-
Caryota—fishtail palms
-
Chamaedorea
-
Chamaerops—European fan palm or Mediterranean fan palm
-
Cocos—coconut palm
-
Corypha—gebang palm, buri palm or tailpot palm
-
Dictyosperma
-
Dypsis
-
Elaeis—oil palms
-
Euterpe
-
Howea
-
Hyophorbe
-
Latania—latan palm
-
Livistona
-
Nannorrhops—mazari palm
-
Phoenix
-
Ptychosperma
-
Rhapidophyllum—needle palm
-
Raphis
-
Roystonea—royal palms
-
Sabal—palmetto or fan palms
-
Syagrus
-
Thrinax
-
Trachycarpus—fan palms
-
Washingtonia
Recorded orchid hosts (Orchidaceae) include the following genera:
-
Acineta
-
Angraecum
-
Anguloa
-
Bletia
-
Brassavola
-
Brassia
-
Brassocattleya
-
Broughtonia
-
Bulbophyllum
-
Cattleya
-
Caularthron
-
Coelogyne
-
Cycnoches
-
Cymbidium
-
Dendrobium
-
Encyclia
-
Epidendum
-
Laelia
-
Maxillaria
-
Miltonia
-
Neofinetia
-
Odontoglossum
-
Oncidium
-
Ornithidium
-
Peristeria
-
Pleurothallis
-
Renanthera
-
Rhynchostylis
-
Schomburgkia
-
Sophronitis
-
Stanhopea
-
Trichopilia
-
Vanda
-
Xylobium
Other recorded host plant families include:
-
Agavaceae
-
Amaryllidaceae—Agave sp.
-
Anacardiaceae—Mangifera indica, mango; Schinus sp., pepper trees
-
Araliaceae—Hedera helix, common ivy
-
Asteraceae—Baccharis sp.
-
Bromeliaceae—Aechmea, Ananas, Aregelia, Billbergia, Bromelia, Catopsis, Guzmania, Neoglaziovia, Pitcairnia, Puya, Ronnbergia, Tillandsia, and Vriesia species
-
Cactaceae
-
Cyperaceae
-
Fabaceae—Acacia, Baikiaea, Cassia, and Leucaena species
-
Heliconiaceae
-
Lauraceae—Persea sp.
-
Liliaceae
-
Moraceae—Ficus sp.
-
Musaceae—Musa sp.
-
Rosaceae—Rosa sp.
-
Rubiaceae—Coffea, Dracaena, and Citrus species
-
Vitaceae—Vitis sp.
A full listing of recorded hosts for boisduval scale is available at https://scalenet.info/
Damage
Boisduval scales are normally found on the leaves and stems of palms. For orchids, this scale tends to prefer the leaf midrib and the portion of the petiole that is covered by the sheath. Boisduval scale is also capable of infesting orchid pseudobulbs and all aerial portions of the plant, including fruit (Miller and Davidson 2005).

Credit: Heidi M. Bowman, UF/IFAS
Management
Various cultural, biological, and chemical control options may be effective for management. Nutritional problems may enhance a host's susceptibility.
Cultural Control
Exclusion is the first measure to be taken to avoid boisduval scale infestation. Carefully examine all plants and propagative materials before purchasing them. However, buyer beware, it is easy to miss scales located underneath leaf sheaths and not all life stages are visible to the unaided eye. New plants should be isolated from collections and nursery stock for at least two weeks to ensure they are pest free. Spacing plants so that their leaves do not touch can help avoid boisduval crawlers moving from infested plants onto clean neighboring plants (Beardsley and Gonzalez 1975). The crawlers can also move from infested to clean plants through strong wind currents in greenhouses and rooms. If possible, isolate infested plants to avoid boisduval scale spread and begin a treatment regimen.
Biological Control
Coccidencyrtus sp. (Hymenoptera: Encyrtidae) have been reported as parasites of boisduval scale (Miller and Davidson 2005, Tenbrink and Hara 1992).

Credit: Avas Hamon, Florida Department of Agriculture and Consumer Services, Division of Plant Industry
Chemical Control
Horticultural oils are often very effective for controlling scale insects, but product labels should be carefully followed. For example, dormant oils applied to the actively growing stage of a plant may result in a burning effect on the plant material. Because crawlers tend to establish themselves on the upper and lower leaf surfaces, near the base of the plant, and in leaf sheaths, thorough spray coverage is important.
For small amounts of plants, using 70% isopropyl alcohol and a cloth or cotton swab to wipe away the scales can provide effective control. However, some soft-leaved orchids may be damaged by isopropyl.
If a chemical application is used, remember that timing the application to target the immature or crawler life stage may be critical for appropriate control. As boisduval scale is an armored scale, the waxy covering may still be present even after it has been effectively killed.
Selected References
Beardsley Jr JW, Gonzalez RH. 1975. The biology and ecology of armored scales. Annual Review of Entomology 20: 47–73.
Buss EA, Dale A. (2016). Scale insects and mealybugs on ornamental plants. MG005. Gainesville: University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/MG005 (6 July 2020)
Cating RA, Hoy MA, Palmateer AJ. 2010. Silwet L-77 improves the efficacy of horticultural oils for control of Boisduval scale Diaspis boisduvalii (Hemiptera: Diaspididae) and the flat mite Tenuipalpus pacificus (Arachnida: Acari: Tenuipalpidae) on orchids. Florida Entomologist 93: 100–106. https://journals.flvc.org/flaent/article/view/76056 (1 March 2022)
Dekle GW. 1965. Arthropods of Florida and Neighboring Land Areas: Florida Armored Scale Insects. Vol.3. Florida Department of Agriculture & Consumer Services, Division of Plant Industry. Gainesville, FL.
Howard FW, Moore D, Giblin-Davis RM, Abad R. 2001. Insects on Palms. CABI Publishing, Wallingford, UK. 400 pp.
Johnson PJ. (November 2001). Scale. American Orchid Society. https://www.aos.org/orchids/orchid-pests-diseases/scale.aspx (6 July 2020)
García Morales M, Denno BD, Miller DR, Miller GL, Ben-Dov Y, Hardy NB. 2016. ScaleNet: A literature-based model of scale insect biology and systematics. Database. doi: 10.1093/database/bav118. https://scalenet.info/.
Miller DR, Davidson JA. 2005. Armored Scale Insect Pests of Trees and Shrubs (Hemiptera: Diaspididae). Cornell University Press. Ithaca, NY. 456 pp.
Tenbrink VL, Hara AH. (1992). Diaspis boisduvalli (Signoret). Crop Knowledge Master. http://www.extento.hawaii.edu/kbase/crop/Type/d_boisdu.htm (7 July 2020)