The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
West Indian marsh grass, Hymenachne amplexicaulis (Rudge) Nees (Poaceae), is a robust, stoloniferous, semiaquatic, perennial grass native to the Neotropics (tropical Central and South America). This perennial grass is considered valuable forage in its native range (Tejos 1978, Enriquez-Quiroz et al. 2006). It reproduces from stolons or seeds in areas with fluctuating water levels and can survive long periods of flooding, but only persists along the edges of permanent deep water (Tejos 1980). West Indian marsh grass is especially adapted to low lying freshwater wetlands and flood plains containing high nutrient and sediment influx (Csurches et al. 1999).
In the 1970s and 1980s, Hymenachne amplexicaulis began invading wetlands in Florida (Langeland and Craddock-Burks 1998). Although the introduction pathway of this grass into Florida is uncertain, intentional introduction into Florida is possible due to its high forage value (Antel et al. 1998, Diaz et al. 2009, Kibbler and Bahnisch 1999). Hymenachne amplexicaulis, a Florida Invasive Species Council (formerly Florida Exotic Pest Plant Council) Category I invasive plant, competitively displaces native vegetation in wetland areas due to its aggressive growth patterns during the rainy season (Diaz et al. 2009, FLEPPC 2019).
In 2000, the "Myakka bug," Ischnodemus variegatus (Signoret) (Hemiptera: Blissidae), was first reported causing severe damage to Hymenachne amplexicaulis at Myakka River State Park, Sarasota County, Florida (Brambila and Santana 2004). Ischnodemus variegatus was identified as a new record for the continental United States by the Florida Department of Agriculture and Consumer Services (FDACS) (Halbert 2000). Research was conducted by University of Florida scientists on the biology, host specificity, and potential impact for this newly introduced, exotic insect species.
Synonymy
The taxonomic status of this species was reviewed by Slater (1987) who raised Ischnodemus variegatus (Signoret) from synonymy with Ischnodemus oblongus Fabricius.
Distribution
The native distribution of Ischnodemus variegatus includes Central and South America. Collection records indicate Hymenachne amplexicaulis may be the only host (Baranowski 1979, Slater 1987).
As mentioned above, both Ischnodemus variegatus and Hymenachne amplexicaulis now occur in Florida. In 1988, Hymenachne amplexicaulis was released in Queensland for use as "ponded pasture" (Csurhes 1999).
Description
Members of the genus Ischnodemus are characterized by elongate, parallel sided bodies, closed fore coxal cavities, terete (cylindrical) antennae, a straight apical (tip) margin, and a forewing membrane with a distinctive morphological texture (i.e. the clause and corium are well differentiated) (Slater and Wilcox 1969). Slater (1976) classified this genus as a 'Type I' body shape which includes species with elongate, slender body shape that is usually slightly flattened. The 'Type I' body shape is advantageous for insects living on the stems of grasses.
Laboratory and field observations indicate the 1st through 4th instars are typically found in aggregations while 5th instars and adults are often observed exploring as individuals. If nymphs or adults are disturbed, they secrete a strong odor from the scent glands located in the thorax and abdomen (Diaz et al. 2008).

Credit: Rodrigo Diaz, University of Florida
Adults
Females (7.23 mm in length, ± 0.56, n=28) are larger than males (6.05 mm in length, ± 0.22, n=49) and both genders have a distinctive "M" pattern at the base of the hemelytra. Female sclerites (hardened plates) at the ventral, or top side, tip of the abdomen are triangular in shape. The last sclerites of males are more rounded.

Credit: Rodrigo Diaz, University of Florida

Credit: Rodrigo Diaz, University of Florida
Adult flying is restricted to short hops of a few meters or less. Gravid females mostly walk, possibly due the large size of their abdomens (Diaz et al. 2008).
Eggs
The egg length is approximately 3 mm (0.1 inches). Eggs are laid in masses (averaging 12 eggs per mass, with a range of 1 to 38) between the leaf sheath and the culm (or stem of the plant), preferentially near the node. Newly deposited eggs are white and older eggs turn bright red (Diaz et al. 2008).

Nymphs
Nymphs of Ischnodemus variegatus have five instars. Early instars initially remain aggregated near the site of oviposition, or egg laying. Later instars migrate to tightly appressed spaces between leaves and stems. Fourth and 5th instars are darker in color than early instars.

Credit: Rodrigo Diaz, University of Florida

Credit: Rodrigo Diaz, University of Florida

Credit: Rodrigo Diaz, University of Florida

Credit: Rodrigo Diaz, University of Florida

Credit: Rodrigo Diaz, University of Florida
Life Cycle and Biology
Average total development time from egg to adult is 40 days. Nymphal eclosion (hatching) take an average of 12 days at 30.5°C (86.9°F). The nymphal stage reaches adulthood in an average of 29 days at 30.5°C (86.9°F). The preoviposition period is about seven days at 28°C (82.4°F) (Diaz et al. 2008). Females lay their eggs in tight spaces between the leaf sheath and the stem. After hatching, the first instars remain together near the site of emergence (Diaz et al. 2008).
Scientists developed a temperature-dependent development model to predict the number of generations that Ischnodemus variegateus could complete per year at different locations in Florida. In North Florida, the model predicts that Ischnodemus variegatus can complete two to three generations per year. In South Florida, the predicted number of generations increases to four to five per year (Diaz et al. 2008).
The optimal temperature range for development and survival is between 28°C (82.4°F) and 33°C (91.4°F). These ideal conditions for Ischnodemus variegatus development match with the weather conditions in central Florida from April to October (Diaz et al. 2008).
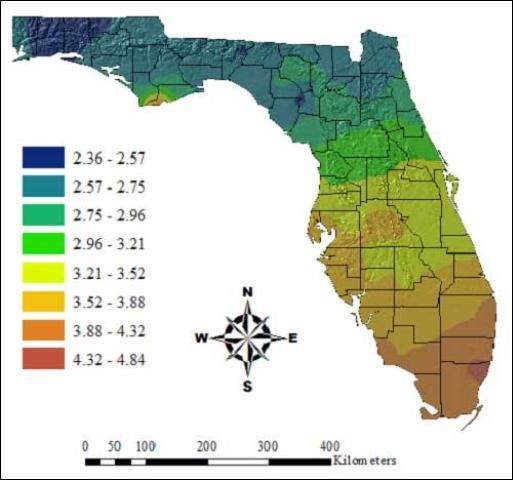
Credit: Rodrigo Diaz, University of Florida
Developmental time and survival of eggs as well as immature stages are affected by temperature. When Ischnodemus variegatus is exposed to low temperatures from 8°C (46.4°F) to 18°C (64.4°F) and to a high temperature of 38°C (100.4°F), low survivorship occurs. Nymphs died within a few days at temperatures exceeding 38°C (100.4°F) and after weeks at lower extreme temperatures, suggesting that Ischnodemus variegatus has a broader lower temperature threshold compared to the upper threshold.
Hosts
Due to economic and ecological importance of grasses, scientists at the University of Florida studied the host range of Ischnodemus variegatus. They found that Hymenachne amplexicaulis is the preferred host of Ischnodemus variegatus in laboratory and field conditions. In laboratory conditions, developmental host range of Ischnodemus variegatus was examined on 57 plant species across seven plant families. Complete development was obtained from Hymenachne amplexicaulis (23.4%), compared to water paspalum, Paspalum repens (Elliott) Kunth (0.4%); beaked panicgrass, Panicum anceps Michx. (2.2%); and fire flag, Thalia geniculata L. (0.3%). In field experiments, Hymenachne amplexicaulis had higher densities of Ischnodemus variegatus than other species (Diaz et al. 2009).

Credit: Rodrigo Diaz, University of Florida
The seasonal cycle of Hymenachne amplexicaulis in Florida begins in spring during seed germination and new shoot growth. Increases in the water level as well as favorable day-length and temperature in the summer allow the grass to grow aggressively. Maximum biomass for Hymenachne amplexicaulis is reached by late summer. Later in the fall, short days trigger flower production (Tropical Weeds Research Centre 2006). During winter, some parts of the grass die, but the stolons (below ground stems) and seeds remain dormant underwater until spring. Based on herbarium specimens collected in the native range, a predictive model of the potential distribution of Hymenachne amplexicaulis in Florida was created suggests that its northern limit in Florida will be Alachua County.
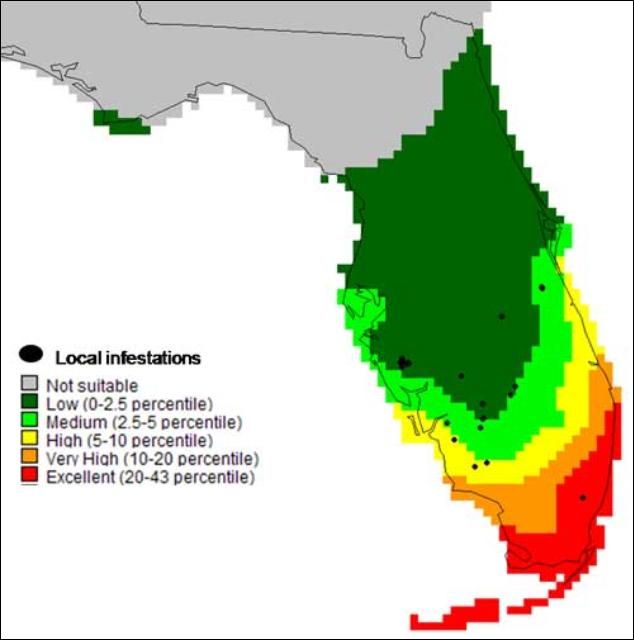
Credit: Rodrigo Diaz, University of Florida
Damage
Damaged leaves turn dark red, due to the accumulation of anthocyanins (a type of pigment in the host plant). Persistent infestations eventually result in leaves turning brown and dying. Feeding effects of Ischnodemus variegatus diminish carbon dioxide assimilation, growth rate, photosynthetic capacity and biomass of Hymenachne amplexicaulis (Overholt et al. 2004). Greenhouse experiments demonstrated that Ischnodemus variegatus feeding damage negatively affected growth of Hymenachne amplexicaulis seedlings (Diaz 2008).

Credit: Rodrigo Diaz, University of Florida
Economic Importance
Population outbreaks of Ischnodemus variegatus during the summer produce a major stress on West Indian marsh grass plants growing in poor conditions (shallow canals). However, plants growing in resource rich environments (deep floodplains, high nutrients runoff) can sustain some damage by Ischnodemus variegatus without impact on the plant's reproductive output (Diaz 2008).
Natural Enemies
Ischnodemus variegatus has two natural enemies in Florida: the egg parasitoid Eumicrosoma sp. (Hymenoptera: Scelionidae) and the entomopathogen Beauveria bassiana (Balsamo) Vuillemin (Deuteromycotina: Hyphomycetes). The egg parasitoid was identified as a potentially accidentally introduced, non-native species for North America (T. Nuhn 2005, personal communication). It attacks young and old eggs, and parasitized eggs turn black. Field sampling in Florida demonstrated that the impact of these natural enemies is minimal to Ischnodemus variegatus populations.
Selected References
Antel NPR, Werger MJA, Medina E. 1998. Nitrogen distribution and leaf area indices in relation to photosynthetic nitrogen efficiency in savanna grasses. Plant Ecology 138:63–75.
Baranowski RM. 1979. Notes on the biology of Ischnodemus oblongus and I. fulvipes with descriptions of the immature stages (Hemiptera: Lygaeidae). Annals of the Entomological Society of America 72:655–658.
Brambila J, Santana F. 2004. First records for Ischnodemus variegatus (Hemiptera: Blissidae) in North America. Florida Entomologist 87:585–586.
Csurhes SM, Mackey AP, Fitzsimmons L. (June 1999). Hymenache (Hymenachne amplexicaulis) in Queensland. Queensland Department of Employment, Economic Development and Innovation. https://www.daf.qld.gov.au/__data/assets/pdf_file/0008/71828/IPA-Hymenachne-PSA.pdf (10 February 2022).
Diaz R. 2008. Biology, host specificity and impact of Ischnodemus variegatus, a herbivore of Hymenachne amplexicaulis. Ph.D. thesis, University of Florida, Gainesville, FL. 187 pp.
Diaz R, Overholt WA, Cuda JP, Pratt PD, Fox A. 2008. Temperature-dependent development, survival, and potential distribution of Ischnodemus variegatus (Hemiptera: Blissidae), a herbivore of West Indian marsh grass. Annals of the Entomological Society of America 101:604–612.
Diaz R, Overholt WA, Cuda JP, Pratt PD, Fox A. 2009. Host specificity of Ischnodemus variegatus, a herbivore of West Indian marsh grass (Hymenachne amplexicaulis). BioControl 54: 307–321.
Enriquez-Quiroz JF, Quero-Carrillo AR, Hernandez-Garay A, Garcia-Moya E. 2006. Azuche, Hymenachne amplexicaulis (Rudge) Nees, forage genetic resources for floodplains in tropical Mexico. Genetic Resources and Crop Evolution 53:1405–1412.
FLEPPC. 2019 List of Invasive Plant Species. Florida Exotic Pest Plant Council. Internet: www.fleppc.org (2 May 2019).
Halbert SE. 2000. Entomology section. Trilogy 39 (5). FDCAS-Division of Plant Industry. http://www.doacs.state.fl.us/pi/enpp/triology/archive/00-sep-oct.html#ent (3 February 2010).
Kibbler H, Bahnish LM. 1999. Physiological adaptations of Hymenachne amplexicaulis to flooding. Australian Journal of Experimental Agriculture 39: 429–435.
Langeland KA, Craddock-Burks K. 1998. Identification and Biology of Non-native Plants in Florida's Natural Areas. University Press of Florida, Gainesville. 165 pp.
Nuhn T. 2005. Personal Communication. USDA Agricultural Research Service. Museum Specialist. Systematic Entomology Laboratory. Washington, DC.
Overholt WA, Ewe S, Diaz R, Morgan EC, Moeri OE. 2004. Feeding effects of Ischnodemus variegatus (Hemiptera: Blissidae) on photosynthesis and growth of Hymenachne amplexicaulis (Poaceae). Florida Entomologist 87:312–316.
Slater JA, Wilcox DB. 1969. A revision of the genus Ischnodemus in the neotropical region (Hemiptera: Lygaeidae: Blissinae). Miscellaneous Publications of the Entomological Society of America 6:199–238.
Slater JA. 1976. Monocots and chinch bugs: a study of host plant relationships in the lygaeid subfamily Blissinae (Hempitera: Lygaeidae). Biotropica 8:143–165.
Slater JA. 1987. The taxonomic status of Ischnodemus oblongus (Fabricius) and Ischnodemus variegatus (Signoret) (Hemiptera: Lygaeidae: Blissinae). Journal of the New York Entomological Society 95:294–297.
Tejos MR 1978. Effect of age on the productivity of paja de agua grass (Hymenachne amplexicaulis (Rudge) Nees) in a controlled flooding savannah. Agronomia Tropical 28:613–626.
Tejos MR. 1980. Production of water straw grass (Hymenachne amplexicaulis (Rudge) Nees) during a savanna period. Congreso Venezolano Zootecnia Guanare (Venezuela) p. 24.
Tropical Weeds Research Centre. 2006. Control methods and case studies, Hymenachne amplexicaulis. Queensland Department of Natural Resources, Minas and Water. http://resourceeconomics.cqu.edu.au/FCWViewer/getFile.do?id=7443 (3 February 2010).