The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Florida has only a few terrestrial slug species that are native (indigenous), but some non-native (nonindigenous) species have successfully established here. Many interceptions of slugs are made by quarantine inspectors (Robinson 1999), including species not yet found in the United States or restricted to areas of North America other than Florida. In addition to the many potential invasive slugs originating in temperate climates such as Europe, the traditional source of invasive molluscs for the US, Florida is also quite susceptible to invasion by slugs from warmer climates. Indeed, most of the invaders that have established here are warm-weather or tropical species. Following is a discussion of the situation in Florida, including problems with slug identification and taxonomy, as well as the behavior, ecology, and management of slugs.
Biology
Slugs are snails without a visible shell (some have an internal shell and a few have a greatly reduced external shell). The slug life-form (with a reduced or invisible shell) has evolved a number of times in different snail families, but this shell-free body form has imparted similar behavior and physiology in all species of slugs. For example, slugs are not as dependent on calcium-rich environments as are shell-bearing snails, but as a result of lacking a protective shell they display behaviors that conserve moisture, such as nocturnal activity and dwelling mostly in sheltered environments. Slugs also reduce water loss by opening their breathing pore (pneumostome) only periodically instead of having it open continuously. Slugs produce mucus (slime), which allows them to adhere to the substrate and provides some protection against abrasion, but some mucus also has chemical properties that function in defense against predation (South 1992).

Credit: Lyle J. Buss, UF/IFAS
Most slugs are hermaphroditic, possessing both male and female sex organs. Thus, at least in some species, a single individual can inseminate another slug, can be inseminated by another, and can even inseminate itself! This makes slugs particularly dangerous as invaders because even a single individual that escapes detection can establish a population in a new environment through self-fertilization. Eggs are white or translucent (though sometimes taking on a yellowish or orange-yellow tint later in egg development) and often nearly spherical. They usually are laid in clusters, either in the soil or similar locations that conserve moisture and may be interspersed with mucus secretions. Eggs are not very resistant to desiccation, so they must remain in fairly humid environments, and absorb some water if hatching is to be successful. Some slugs deposit fecal-like material with their eggs, but the reason for this behavior is unknown. Development time of slugs varies with weather conditions and among species, but several months or more are commonly required for slugs to reach maturity. Slugs often develop faster and commence reproduction sooner under warm conditions but attain a larger size and ultimately produce the same number or more eggs in cooler conditions.

Credit: Lyle J. Buss, UF/IFAS
Slugs tend to have omnivorous dietary habits. Many feed on fungi, decomposing vegetation, and soil, as well as living plant tissue. They are opportunistic, so their diets often reflect what is available, as well as innate and learned preferences. Some slugs thrive in disturbed habitats linked to human activity (anthropogenic), establishing the potential to become pests of agricultural or ornamental plants. Their nocturnal habits and ability to burrow into the soil make them difficult to detect. Their mouth contains a rasping structure called a radula, which bears tooth-like features, but these are internal and not generally visible. Young slugs may feed only on the surface of vegetation, but larger slugs remove entire sections of foliage, leaving irregular holes in foliage, flowers, and other soft plant tissue.

Credit: John L. Capinera, UF/IFAS

Credit: John L. Capinera, UF/IFAS
Slugs can be quite long-lived, surviving for a year or more. Unlike some invertebrates such as insects, they can continue to grow after they reach reproductive maturity and commence egg production. Thus, their adult size is quite variable. Their eggs are not all produced in a single batch; instead, they are deposited periodically in soil or leaf litter.
Economic Importance
Because slugs are omnivorous, if conditions are favorable, they feed on plants, sometimes becoming pests in gardens, nurseries, and crops. They also have regulatory significance, interfering with movement of potted plants, because locations lacking slugs are not eager to be inoculated with these potentially damaging species. Florida's generally sandy soil is not conducive to slugs, but they occur where organic matter is abundant, and of course the generally humid conditions favor slug survival.
A slug that commonly causes damage in Florida is the marsh slug, Deroceras laeve (Müller 1774). This small species apparently is indigenous, or at least is widespread, in North America. Deroceras laeve become most active and damaging during the cooler, wet conditions of spring and early summer and do not cause much foliage damage during the hotter months of summer, even when moisture is abundant. Damage by Deroceras laeve often goes undiagnosed because, like all slugs, they feed only at night. Heavily mulched planting beds provide excellent harborage for this slug, including shelter during the daylight hours. Flower and foliage plants suffer the most damage in Florida, but this slug attacks many different plants. A large species known as Florida leatherleaf slug, Leidyula floridana (Leidy 1851), also inflicts injury to plants, though this species usually is not abundant.
Although not usually considered to be important pests in Florida, elsewhere in the US some of the introduced European slugs have caused great damage to many vegetable crops, field crops, and ornamental plants. Some may transmit plant disease-causing organisms such as viruses and fungi, or serve as intermediate hosts of animal parasites, such as tapeworms and lung worms (Godan 1983). Slugs also are a threat to animals and people because they serve as intermediate hosts of the nematode Angiostrongylus costaricensis, also known as rat lungworm, which causes a disease called human abdominal angiostrongyliasis (Rueda et al. 2004). Although outbreaks of this rodent-associated disease occur in Central America, it occurs infrequently in the United States. The nematode now resides in many molluscs found in Florida, however, so the threat of disease is real (Capinera and Walden 2016).
Several potentially damaging slugs have been intercepted in commerce but apparently failed to establish in Florida. These include:
- black arion, Arion ater (Linnaeus 1758)
- brownbanded arion, Arion circumscriptus (Johnston 1828)
- gray field slug, Deroceras reticulatum (Müller 1774)
- yellow garden slug, Limacus flavus (Linnaeus 1758)
- giant garden slug, Limax maximus (Linnaeus 1758)
- greenhouse slug, Milax gagates (Draparnaud 1801)
Other species of slugs that have not been intercepted in Florida, but which threaten, include
- Spanish slug, Arion vulgaris (lusitanicus) (Moquin-Tandon 1855)
- Cuban slug, Veronicella cubensis (L. Pfeiffer 1840)
- bean slug, Sarasinula plebeia (Fischer 1868)
- pancake slug, Veronicella sloanei (Cuvier 1817)
Management
For the most part, our native fauna remain in natural, undisturbed habitats where they function mostly as decomposers, rarely achieving pest status. However, nonindigenous slugs are increasing in visibility and importance as pests. Primarily due to international trade, nonindigenous slugs continue to be introduced to North America, so other species are likely to establish populations in Florida. Therefore, management of slugs in Florida will likely be more of a concern in the future.
Slug control and management have been reviewed by many authors (e.g., Henderson and Triebskorn 2002; Bailey 2002). The management tools for slugs are much the same as used in the integrated pest management (IPM) strategies for other invertebrate pests, such as insects. However, development of chemicals and research on biological control (i.e., potential predators and parasites) have been done mostly outside of the US, and options for management are more limited than with insects.
A comprehensive review of the natural enemies of terrestrial slugs can be found in Barker (2004). Natural enemies are relatively few. Some birds, especially ducks, feed on slugs. Moles and shrews also will feed on slugs. Few predaceous insects attack slugs, but in Coleoptera the larvae of Lampyridae (fireflies) and adult Carabidae (ground beetles) do so occasionally. Some parasitic flies (Diptera, especially marsh flies, family Sciomyzidae and phorid flies, family Phoridae), a few fungi, and many protozoa are known to affect slugs (Godan 1983). Phasmarhabditis hermaphrodita (Nematoda: Rhabditida), a slug-parasitic nematode used in Europe to control slugs, has not been allowed in the US because of concern about introducing a non-native organism. However, P. hermaphrodita was recently detected in the US (De Ley et al. 2014), so perhaps it will become commercially available here. Other nematodes found in the US have been investigated for slug control, but the results were not encouraging.
Only recently, New Guinea flatworm, Platydemus manokwari De Beauchamp (1963) (Platyhelminthes: Tricladida: Geoplanidae) was found in Florida. A native of New Guinea, it has been accidentally spread to many locations, mostly in the Pacific region. It kills mostly snails but also slugs. In addition to threatening some native molluscs, it also is a host of rat lungworm.
Predatory snails such as the rosy wolf snail, Euglandina rosea (Férussac 1821), will attack slugs, and may account, in part, for the relatively low slug densities in Florida. Euglandina rosea prefers snails to slugs but will attack and consume small slugs in the absence of snail prey. Euglandina rosea is native to the southeastern US and is quite common in woodlands and gardens in Florida. It has been relocated to other parts of the world, including Hawaii, India, and many islands in the Pacific region, in an attempt to control invasive snails such as giant African land snail, Achatina fulica (Férussac 1821). It has been used to provide partial control of giant African snail, but it has been quite disruptive to native snail populations, so its use is discouraged outside its natural range (Barker 2004).

Credit: Lyle J. Buss, UF/IFAS
Slugs benefit from having shelter such as plant debris, so removal of boards, rubbish, piles of brush, and other debris will help limit slug numbers. On the other hand, strategic placement of such shelter can be used to sample or even reduce slug populations if the slugs are removed and destroyed periodically.
Moisture is also an important factor governing slug distribution and activity. Dense vegetation, deep mulch, and frequent irrigation favor slugs. Consequently, minimizing irrigation (especially overhead sprinkling) or planting drought-tolerant vegetation may reduce slug problems.
When chemical control is needed, commercial slug and snail baits are usually used by scattering bait around vegetation that is to be protected. The bait contains a toxicant, of course. Traditionally, the toxicant in such baits is metaldehyde, sometimes metaldehyde plus a carbamate toxicant, or occasionally a carbamate toxicant alone. These are effective, but quite toxic, and they pose a threat to non-target organisms such as pets and vertebrate wildlife. Increasingly, they are unavailable.
More recently, baits with iron-based toxicants such as iron phosphate and sodium ferric EDTA have been shown to be effective toxicants when applied to slugs and snails and are safer to use. Boric acid and sulfur-based products have been less effective in the small number of studies that have been conducted. Regardless of the toxicant, baits should be scattered sparsely in and around vegetation, so as to make it unlikely that pets or wildlife will ingest too much of the bait. If possible, it is a good idea to irrigate prior to bait application because the additional moisture will stimulate increased slug activity, increasing the likelihood that they will eat the bait. Poisoned slugs typically display loss of coordination and paralysis, and increased mucus secretion. They often die from desiccation following paralysis, but detoxification of the poison by the slug is sometimes accomplished, so they may recover and survive (Henderson and Triebskorn 2002). Diatomaceous earth is sometimes recommended for slug control because of its purported abrasiveness, but there is no scientific evidence to support its use for slugs.
Ideally, a non-toxic repellent or feeding deterrent would be available to protect vegetation, without introducing more toxicants into the environment. Two products have recently become available, both based on essential oils extracted from plants. Slug & Snail Defense™ contains a mixture of plant essential oils and pepper, but in tests with Leidyula floridana there was no protection of leaf material. On the other hand, following exposure to Snail and Slug Away™, which contains cinnamon oil as the active ingredient, considerable reduction of plant feeding was noted. Also, when sprayed directly on Leidyula floridana slugs, Snail & Slug Away caused rapid mortality. Finally, although not labeled for management of molluscs, incidental benefits of copper hydroxide fungicide are well documented. If you are applying copper-based fungicides to your plants to control fungi or bacteria, you can anticipate that feeding by slugs on treated foliage will be greatly reduced. Copper hydroxide products with high concentrations of copper (about 60%) are most effective (Capinera and Dickens 2016).
Identification of Slugs Found in Florida
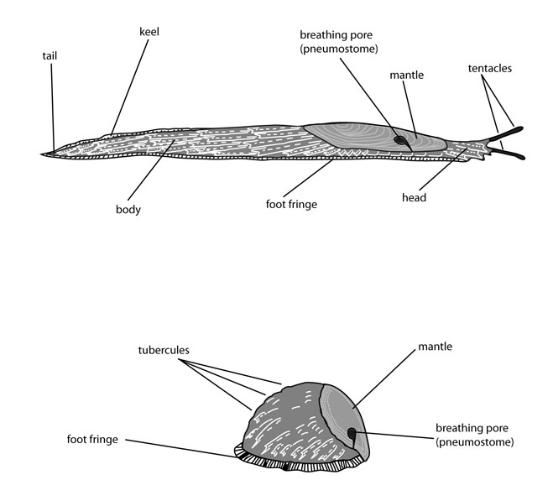
Superficially, most slugs appear to be quite similar, with a naked, unsegmented body covered by mucus and bearing two pairs of tentacles anteriorly, one of which bears the eyes (Figures 1 and 6). However, closer examination reveals major differences among some groups, and the slugs now in Florida are typical of this pattern. For example, veronicellid slugs are rather flattened, and have a narrow foot, and so they are fairly distinct from other slugs. However, within a slug family there can be considerable uncertainty about correct identifications and speciation.
Because of the many errors in the literature involving snail and slug identification, it can be misleading to rely entirely on a literature review to assess molluscan fauna. Color is not a reliable means of identification, as some species have more than one color form. Clearly, external morphology alone is not always a reliable way to identify slugs, especially to the species level. Often it is necessary to use a combination of external traits (morphology), internal anatomy (especially reproductive structures), and even molecular diagnoses (DNA analysis) for species-level determinations.
Key to the Families
Following is a simple key to the families of slugs found in Florida. The variation in appearance among individual slugs makes it very difficult to identify some species with great certainty, especially when working with individual specimens. However, many species can be identified with a reasonable degree of confidence if you collect several individuals so you can assess their variation, and it is also usually necessary to collect adults.
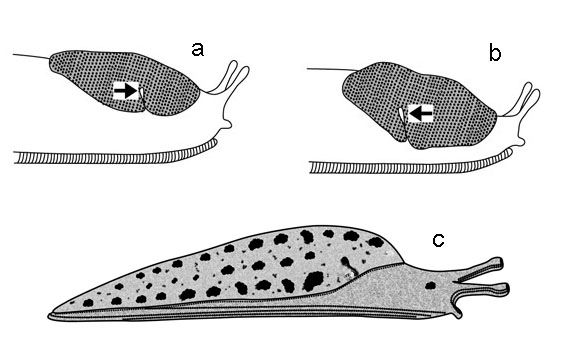
1. Mantle covering all of the back (dorsum) of the animal, or nearly so, or saddle-like mantle structure not apparent in anterior region when body is extended (Figures 15–18); breathing pore (pneumostome) not visible or located anteriorly (toward the head) on right margin of mantle (although it may be closed) (Figure 7) . . . . . . 2
1'. Mantle consisting of an elevated saddle-like structure that is apparent when the body is extended, and located only in the anterior region of the body (Figures 19–23); breathing pore (pneumostome) present (although it may be closed) posteriorly (away from the head) on right margin of mantle (Figure 7) . . . . . . 3
2(1). Body circular or oval in cross-section and not tapering laterally (toward the sides) (Figure 10); breathing pore (pneumostome) found near anterior right mantle edge; foot nearly as wide as body (Figure 11)—Family Philomycidae, the mantleslugs 2'. Body rather flattened in cross-section, and tapering laterally (toward the sides); breathing pore (pneumostome) not visible (Figures 15–18); foot considerably narrower than width of body (Figures 8 and 9)—Family Veronicellidae, the leatherleaf slugs

Credit: Lyle J. Buss, UF/IFAS
3(1'). Ridges on mantle forming fingerprint-like pattern that is not centered dorsally, rather being offset slightly to the right side of the animal—Family Agrolimacidae 3'. Ridges on mantle forming fingerprint-like pattern that is centered dorsally (Figures 22 and 23)—Family Limacidae, the keelback slugs
Florida Species by Family
Family Philomycidae, the Mantleslugs
Carolina Mantleslug, Philomycus carolinianus (Bosc, 1802)
This large slug has its mantle extending the entire length of its body. It can be 3–10 cm (1.2 to 3.9 in) long at maturity. Typically, it is tan with brown or black spots and blotches. In addition, there may be two rows of dark spots along the back (dorsum) (Figure 10). However, this slug can be somewhat variable in appearance and sometimes is fairly pale or mostly dark. The foot is nearly as wide as the body (Figure 11). The breathing pore (pneumostome) is located in a lightly pigmented anterior right area of the mantle (Figures 12 and 13). This slug can grow to weigh 8 g, though it becomes sexually mature and begins egg production when it is about 3 g. This species feeds on fungi. It is commonly found under logs, loose bark, and aerial bromeliads. This species normally is found in woodlands and does not frequent disturbed habitats like the veronicellid slugs. The Carolina mantleslug is a native species, found from Maine to Florida, and west to Iowa and Texas. Named subspecies exist, and it may be comprised of a species complex.

Credit: Lyle J. Buss, UF/IFAS
Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS
An entirely albino or tan-colored form of Carolina mantleslug (Figure 14) occurs in southernmost Florida, where it lives under loose bark and feeds on fungi growing on partly submerged trunks of dead hardwood trees. Like the more common forms, it is a large slug, attaining up to 8 g (0.28 oz) in weight and 10 cm (3.9 in) in length.

Credit: Lyle J. Buss, UF/IFAS
Foster Mantleslug, Pallifera fosteri (F.C. Baker 1939)
This is a very small slug, less than 1 cm (0.4 in) long. Its mantle covers only the posterior 2/3 of the animal and is thicker in the anterior region of the mantle, giving the slug a hump-backed appearance (Figure 7c). Its background color is whitish or tan, but it bears numerous black spots. The spots sometimes coalesce to form blotches and may also form an interrupted irregular line laterally on the mantle. This slug occurs widely in the eastern US, but in Florida it is documented only from Marion County. Due to its small size and preferred habitat (deciduous woodlands), it may well be more broadly distributed but overlooked. In Illinois, it is reported to occur under loose bark, often in association with Philomycus carolinianus.
The related but larger Megapallifera mutabilis (Hubricht 1951), also known as the changeable mantleslug, is known from counties in Alabama adjacent to Florida and may occur in the Panhandle region of Florida but so far is unreported there.
Family Veronicellidae, the Leatherleaf Slugs
Black-Velvet Leatherleaf, Belocaulus angustipes (Heynemann 1885)
Adults of this species are uniformly black in color dorsally and velvety in appearance (Figure 15), with the underside paler in color. The velvety black color of these slugs occasionally is interrupted by a pale median stripe, especially in juveniles. The very young slugs are not so darkly colored. Because of this black velvety appearance, it is unlikely to be confused with any other slug found in Florida. It can attain a length of 5 cm when extended, but is not a large slug, attaining a weight of about 1.2 g (0.04 oz) at maturity. Like most slugs, B. angustipes is usually seen only during wet weather, spending most of its life foraging at night in leaf litter or in the soil. A native of South America, this species is now established in the states that border the Gulf of America, formerly Gulf of Mexico (Walls 2009), including northern and central Florida. It occurs in greenhouses and nurseries, where it can be found under potted plants, so it is destined to be spread further with nursery stock. The slug also burrows in soft soil and can enter the root-balls of plants through drainage holes at the base of the containers. This species reportedly eats both living and decayed leaves, although it is not considered to be a pest in Florida because it is not abundant. Sometimes it is found in lawns (Walls 2009) and disturbed areas near homes, such as drainage ditches.
Early references refer to this species either as Angustipes ameghini (Gambetta 1923) or Veronicella ameghini (Gambetta 1923).

Credit: Lyle J. Buss, UF/IFAS
Florida Leatherleaf, Leidyula floridana (Leidy 1851)
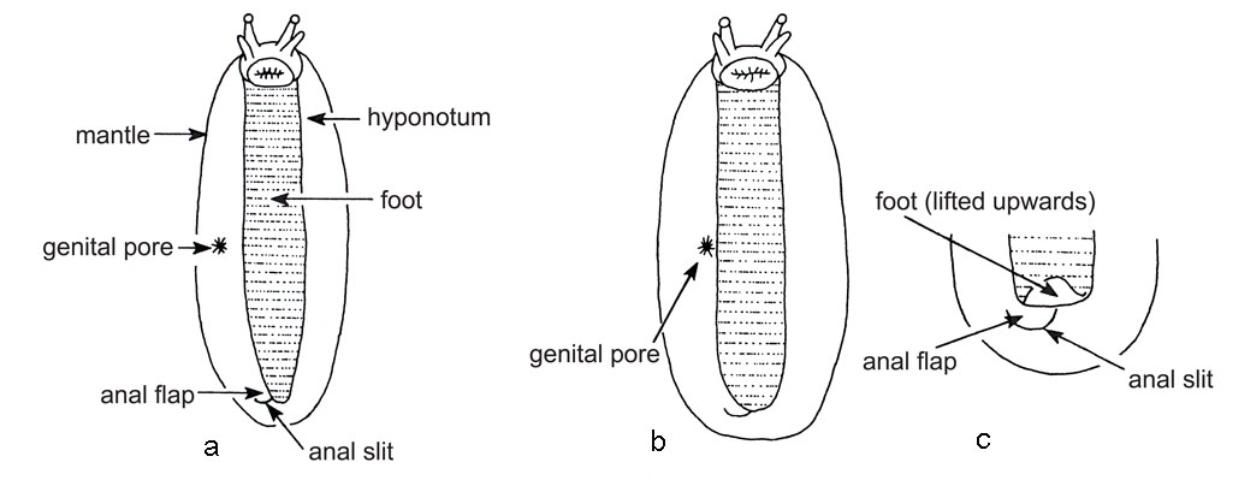
This slug is tan dorsally and mottled with brown or black spots that often coalesce into dark dorsolateral stripes (Figures 16 and 17), and bearing a long, pale medial stripe. However, the color pattern can be quite variable. The genital pore is located adjacent to the foot (Figure 8), normally less than 1/4 hyponotal width (the hyponotum is the portion of the mantle that wraps beneath the slug body and is adjacent to the foot) from the foot. The anal slit (Figure 12) usually is visible, extending beyond the right edge of the retracted foot. It is native to the Caribbean (Cuba to Jamaica) and south Florida. Formerly found only in southern and central Florida, it has since been spread to northernmost Florida, and also is found in Louisiana, Texas, and northeastern Mexico, suggesting either that the species is more widespread than previous records indicated or that it is being relocated via commerce. Normally, it is the most commonly encountered veronicellid in Florida. No serious economic damage has been reported thus far from Florida, although they feed readily on both crop and ornamental plants and can be a nuisance. These slugs can attain a weight of 12 grams (0.4 oz) and measure over 5 cm (2 in) in length.
Leidyula floridana has also been known as Vaginulus floridanus (Tate 1870) and Veronicella floridana (Leidy 1851).

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS
Other Veronicellids Threatening Florida
Other veronicellids have been found in Florida or may soon become established. One is Veronicella sloanei (Cuvier 1817), also known as the pancake slug. The pancake slug is large, often 5–12 cm (2–4.7 in) in length, and usually very pale, ranging from whitish to tan or speckled with brown spots. Especially in juveniles, their body may bear two dorsolateral stripes extending from behind the antennae backward along the body, though they are rather diffuse. The eyestalks are bluish. In Florida, this species has been observed only in Miami-Dade County, and it is uncertain whether it persists. Elsewhere, the pancake slug is found throughout the Caribbean and on some islands in the Pacific, including Hawaii. It has a very wide host range, including many vegetables and ornamental plants.
Another significant threat is Veronicella cubensis (L. Pfeiffer 1840), known as the Cuban slug. This slug is 5–12 cm (2–4.7 in) long and variable in color, but usually brown with dark but thin dorsolateral stripes along its back and a thin light-colored stripe dorsally. It is very difficult to distinguish from the Florida leatherleaf slug. Like the pancake slug, it occurs widely in the Caribbean and the Pacific, where it feeds on numerous crop and ornamental plants. It has been intercepted in Florida and apparently is established in Santa Barbara County, California.
Family Agrolimacidae
Marsh Slug, Deroceras laeve (Müller 1774)
This is the smallest of the common slugs in Florida, weighing as little as 0.2 g (0.007 oz) at maturity, but up to 0.8 g (0.03 oz). It is brownish or grayish, without spots or stripes, and bears only indistinct markings, often including minute white flecks (Figures 18 and 19). Though only 1 cm (0.4 in) long at rest, the marsh slug can become more elongate (up to 3 cm (1.2 in)) when extended. The eggs are deposited in small clusters (often six to 10, but up to 33) in soil or organic detritus. The eggs initially are transparent but become yellowish as they mature, usually hatching in two to three weeks (Faberi et al. 2006). They are 1–3 mm (0.04–0.1 in) long and vary from round to oval in shape. This species is found widely in North America but also occurs in Europe, Asia, and South America. In Florida, it is found from the Keys to Pensacola, and feeds on a great number of plants in cultivated areas as well as in swamps, woodlands, and around human habitations. Although tolerant of a wide range of environmental conditions, in Florida it is inactive during the heat of the summer. Its temperature tolerances appear to be broader than that of the gray field slug, Deroceras reticulatum (Getz 1959), a much more damaging species in North America. This attribute likely explains the presence of the marsh slug in Florida but the absence of the gray field slug.
Although formerly a bit confused, the scientific name and identity of Deroceras laeve have been stable for many years.

Credit: William Leonard, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS
Family Limacidae, the Keelback Slugs
Banded Slug, Lehmannia valentiana (Férussac 1822)
This slug (also known as threeband garden slug) is larger than Deroceras laeve, attaining a length of 5–7 cm (2–2.8 in), although it begins reproduction when considerably smaller, about 2.5 cm (1 in) long and 1.2 g (0.04 oz) in weight. It is light brown or reddish brown with a pair of dark dorsolateral stripes extending over the mantle and body (Figures 21–23). Despite the name 'keelback' being applied to this family, this species shows little or no evidence of a keel (dorsal ridge on the tail). Originally from southwestern Europe, it has been introduced to many states in the US, from New York to California and Hawaii, but in northern areas it is found mostly in greenhouses (Skujiené 2002). It deposits clusters containing up to 63 eggs that hatch in about 14 days. They measure 2.0–2.5 mm (0.08–0.1 in) in length and are oval in shape. Like other problem slugs, it is anthropogenic and often found near human habitations. In Florida, it is established only in the Pensacola area. It has also been introduced to many other countries, including Australia, New Zealand, some Pacific islands, and regions of South America.
The name of this species has changed repeatedly (e.g., Limax valentianus [Férrusac 1823], Limax poirieri [Mabille 1883]), and most North American records for this species refer to it as Lehmannia marginata (Hoffman 1928).

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS

Credit: Lyle J. Buss, UF/IFAS
Selected References
Auffenburg K, Stange LA. (2008). Snail-eating snails of Florida (Euglandina rosea (Férrusac 1821), Rumina decollata (Linnaeus 1758), Haplotrema concavum (Say 1821), Gulella bicolor (Hutton 1834), Varicella gracillima floridana (Pilsbry 1907). Featured Creatures. https://edis.ifas.ufl.edu/publication/IN523
Bailey SER. 2002. Molluscicidal baits for control of terrestrial gastropods. Pages 33–54 in Barker GM (ed.). Molluscs as Crop Pests. CABI Publishing, Wallingford, UK.
Barker GM (ed.). 2001. The Biology of Terrestrial Molluscs. CABI Publishing, Wallingford, UK. 558 pp.
Barker GM (ed.). 2002. Molluscs as Crop Pests. CABI Publishing, Wallingford, UK. 468 pp.
Barker GM (ed.). 2004. Natural Enemies of Terrestrial Molluscs. CABI Publishing, Wallingford, UK. 644 pp.
Capinera JL, Dickens K. 2016. Some effects of copper-based fungicides on plant-feeding terrestrial molluscs: A role for repellents in mollusc management. Crop Protection 83: 76–82.
Capinera JL, Guedes Rodgrigues C. 2015. Biology and control of the leatherleaf slug Leidyula floridana (Mollusca: Gastropoda; Veronicellidae). Florida Entomologist 98: 243–253.
Capinera JL, Walden HS. 2013. Rat lungworm, Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Strongylida: Metastrongylida). UF/IFAS Department of Entomology and Nematology, EENY 570. https://edis.ifas.ufl.edu/publication/IN1007
Cowie RH, Dillon RT Jr, Robinson DG, Smith JW. 2009. Alien non-marine snails and slugs of priority quarantine importance in the United States: a preliminary risk assessment. American Malacological Bulletin 27: 113–132.
Deisler JE, Stange LA. 1984. The verocellid slugs of Florida (Gastropoda: Veronicellidae) Florida Department of Agriculture and Consumer Services Entomology Circular 261. 4 pp.
De Ley IT, McDonnell RD, Lopez S, Paine TD, De Ley P. 2014. Phasmarhabditis hermaphrodita (Nematoda: Rhabditidae), a potential biological control agent isolated for the first time from invasive slugs in North America. Nematology 16: 1129–1138.
Faberi AJ, López AN, Manetti PL, Clemente NL, Álvarez Castillo HA. 2006. Spanish Journal of Agricultural Research 4: 345–350.
Garcia EN, Thomé JW, Castillejo J. 2007. A review of the Veronicellidae from Mexico (Gastropoda: Soleolifera). Revista Mexicana de Biodiversidad 78: 41–50.
Getz LL. 1959. Notes on the ecology of slugs: Arion circumscriptus, Deroceras reticulatum, and D. laeve. American Midland Naturalist 61: 485–498.
Henderson I, Triebskorn R. 2002. Chemical control of terrestrial gastropods. Pages 1–31 in Barker GM (ed.). Molluscs as Crop Pests. CABI Publishing, Wallingford, UK.
Jacksonville Shell Club. Northeast Florida slug page. https://www.jaxshells.org/807b.htm (1 June 2011).
Karlin EJ, Bacon C. 1961. Mating, and egg-laying behavior in the Limacidae (Mollusca). Transactions of the American Microscopical Society 80: 399–406.
Rueda A. 1989. Biology, Nutritional Ecology, and Natural Enemies of the Slug Sarasinula plebeia (Fischer,1868) (Soleolifera: Veronicellidae). Unpublished M.S. Thesis, University of Florida, Gainesville. 163 pp.
Rueda A, Caballero R, Kaminsky R, Andrews KL. 2004. Vaginulidae in Central America, with emphasis on the bean slug Sarasinula plebeia (Fischer). Pages 115–144 in Barker GM (ed.). Molluscs as Crop Pests. CABI Publishing, Wallingford, UK. 468 pp.
Robinson DG. 1999. Alien invasions: the effects of the global economy on non-marine gastropod introductions into the United States. Malacologia 41: 413–438.
South A. 1992. Terrestrial Slugs: Biology, Ecology and Control. Chapman and Hall, London. 428 pp.
Skujiené G. 2002. Lehmania valentiana (Férussac 1823) — a newly introduced slug species in Lithuania (Gastropoda: Pulmonata: Limacidae). Act Zoologica Lituanica 12: 341–344.
Walls JG. 2009. Just a plain black slug: Belocaulus angustipes. American Conchologist 37: 28–29.
White-McLean J, Capinera JL. 2014. Some life history traits and diet selection in Philomycus carolinianus (Mollusca: Gastropoda: Philomycidae). Florida Entomologist 97: 511–522.