The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Diaphorencyrtus aligarhensis (Shafee, Alam and Argarwal 1975) (Hymenoptera: Encyrtidae) is a host-specific, solitary endoparasitoid of Diaphorina citri Kuwayama (Hemiptera: Psyllidae), the Asian citrus psyllid (ACP). Diaphorina citri is a serious pest throughout citrus growing regions of the world (Da Graca 1991, Halbert and Manjunath 2004). Psyllid feeding activities cause leaf chlorosis and promote the growth of sooty mold, but more significantly the psyllids can vector the bacterium Candidatus Liberibacter asiaticus, a causal agent of citrus greening disease or huanglongbing (HLB). Infected trees begin to decline, producing yellow shoots, leaf mottling, low quality fruit and may experience mortality (Aubert et al. 1996, Bové 2006). Diaphorina citri was discovered in south Florida in 1998 (Halbert 1998), while citrus greening first appeared in 2005 (Halbert 2005).
In addition to utilizing chemical and mechanical methods to control both the Asian citrus psyllid and huanglongbing, the biological control agents Tamarixia radiata (Waterston) (Hymenoptera: Eulophidae) and Diaphorencyrtus aligarhensis were imported into Florida and released (Hoy and Nguyen 2000). Both parasitoid species have been credited with reducing psyllid populations in Réunion (Etienne and Aubert 1980) and Taiwan (Chien and Chu 1996).
Synonymy
Ruggiero et al. (2011) reviewed past taxonomic names published for Diaphorencyrtus aligarhensis including:
Aphidencyrtus aligarhensis
Aphidencyrtus diaphorinae
Aphidencyrtus sacchari
Diaphorencyrtus diaphorinae
Psyllaephagus diaphorinae
Syrphophagus aligarhensis
Distribution
Diaphorencyrtus aligarhensis was originally recorded from India (Shafee et al. 1975) and later from the Philippines, Vietnam (Aubert 1987), and China (Yang et al. 2006). Specimens were imported and released for classical biological control programs in Réunion, Taiwan, and the United States (Etienne and Aubert 1980, Chien, 1995, Hoy and Nguyen 2000).
Description
Adults
Adult wasps are small (~ 1-1.5 mm (< 1/16 in)) and have yellow legs and antennae and a black head and thorax. Sexual dimorphism is exhibited in slight differences in the antennae and abdomen. Female antennae are smooth and clubbed while male antennae are slightly longer, lack clubs and are covered with short hairs. Females possess a large, rounded yellow abdomen with a black posterior while male abdomens are smaller, solid black, and more cylindrical.

Credit: Eric Rohrig, University of Florida.
Eggs
Eggs are oval, with an average length of 130 ± 10 µm and a diameter of 100 ± 1 µm. Newly laid eggs are milky white but begin to clear within 12–24 hours. After clearing, the developing embryo can be seen through the clear smooth chorion.
Larvae
All larval stages are hymenopteriform with a weakly developed head capsule. Larvae lack hairs or spiracles and have short, small, reddish-orange mandibles that are approximately 20% of the total body width. First instar larvae average 410 ± 7 µm long by 100 ± 5 µm diameter and increase in size to 1190 ± 10 µm long by 400 ± 20 µm diameter by the fourth larval instar. Larvae entering prepupation shorten in length by constriction.
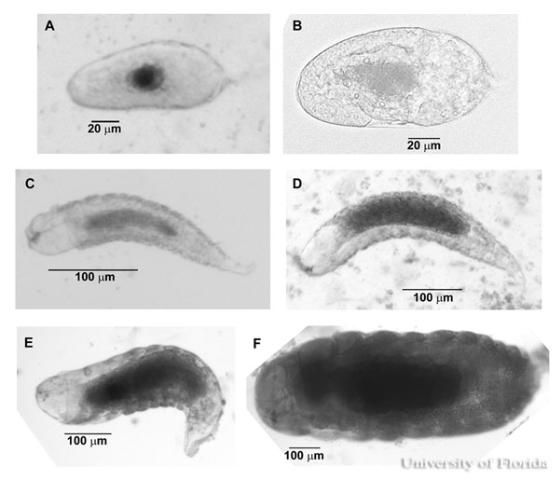
Credit: Eric Rohrig, University of Florida
Pupae
Pupating larvae begin to develop adult head and abdomen definition first, followed by legs, mouthparts, and antennae. Next, the thorax, eyes, and ocelli begin melanization, followed by the abdomen, legs, and lastly the antennae.

Credit: Eric Rohrig, University of Florida
Life Cycle
Diaphorencyrtus aligarhensis generally reproduces by thelytoky, which results in all female offspring (Chien 1995, Hoy 2003). Imported wasps tested positive for infection with the bacterial endosymbiont Wolbachia through PCR analysis (Jeyaprakash and Hoy 2000, Rohrig et al. 2011). Wolbachia infection can induce parthenogenesis as well as other reproductive anomalies in insects (Werren 1997). Shafee et al. (1975) reported small numbers of males in Asian populations surveyed.
Female Diaphorencyrtus aligarhensis oviposit a single egg into the host, usually through its abdomen. If two wasps lay an egg in the same host nymph (superparasitism), only one will complete development to adulthood (Rohrig et al. 2011). Diaphorencyrtus aligarhensis are considered koinobiont parasitoids because host Diaphorina citri nymphs continue to feed and develop following a parasitism event until they are eventually killed before reaching adulthood.
Females parasitize 2nd through 4th instar nymphs and host feed on 1st through 4th instars (Chien and Chu 1996, Skelley and Hoy 2004). Adult females are capable of killing up to 280 Diaphorina citri nymphs through host feeding and parasitism combined (Chien 1995). Skelley and Hoy (2004) reported that there was no significant difference in the mean number of progeny or adult wasp size when reared from 2nd, 3rd or 4th instar nymphs. Females produced an average of 6.6 progeny per day during laboratory rearing and only 10% of adult Diaphorencyrtus aligarhensis could survive for 50 days at 25°C (77°F) (Skelley and Hoy 2004). Chein (1995) determined mean longevity at 20 days.

Credit: Eric Rohrig, University of Florida

Credit: Eric Rohrig, University of Florida
Life cycle from egg to adult requires 16 to 18 days at 25°C (77°F) (Rohrig et al. 2011, Chien 1995). Diaphorencyrtus aligarhensis development includes an embryonic stage (~ 2 day duration), four larval instars (~ 6 days), a prepupal (~ 1 day), and a pupal stage (~ 7 days) that occur within Diaphorina citri nymphs. Parasitized nymphs die and begin to harden into a brown mummy as the developing parasitoid nears the end of the larval stage of development (~ 8 days post parasitism).
During pupation, Diaphorencyrtus aligarhensis develops with its ventral surface toward the nymph's ventral surface and its anterior end toward the nymph's posterior end. Upon reaching adulthood, the wasp rolls 180° so that it is now facing the host's dorsal surface. The adult wasp chews a round exit hole through the nymph's abdomen, crawls out, grooms, moves its wings and begins walking. This exit hole can be used to distinguish between a mummy parasitized by Diaphorencyrtus aligarhensis from Tamarixia radiata which exits dorsally through the thorax (Hoy 2005) rather than the abdomen.

Credit: Eric Rohrig, University of Florida
Hosts
There is no evidence that Diaphorencyrtus aligarhensis is capable of developing on any host other than Diaphorina citri (Aubert and Quilici 1984).
Diaphorencyrtus aligarhensis Use in Biological Control of Diaphorina citri in Florida
After a successful classical biological control program targeting Diaphorina citri was conducted in Réunion, both Diaphorencyrtus aligarhensis and Tamarixia radiata were evaluated for release in the United States.
Diaphorencyrtus aligarhensis was originally imported into Florida from Taiwan in 1998 and field releases began in 2000 (Hoy and Nguyen 2000). Another population of Diaphorencyrtus aligarhensis from Guangdong, China was imported in 2006 and released a year later (Rohrig et al. 2012). Tamarixia radiata from Taiwan and Vietnam were released into Florida beginning in 1999. Currently, Tamarixia radiata has established in most citrus growing regions of Florida, providing varying levels of control (Michaud 2002, Quereshi et al. 2009), while Diaphorencyrtus aligarhensis appears not to have established despite several recoveries.
It is believed that a combination of the superiority of Tamarixia radiata as a biological control agent of Diaphorina citri, increased/more efficient use of pesticides to control Asian citrus psyllids, inconsistent populations of immature psyllids, and predation of parasitized hosts by generalist predators likely impeded the establishment of Diaphorencyrtus aligarhensis (Rohrig et al. 2012).
Selected References
Aubert B. 1987. Trioza erytreae del Guercio and Diaphorina citri Kuwayama (Homoptera: Psylloidea), the two vectors of citrus greening disease: Biological aspects and possible control strategies. Fruits 42: 149–162.
Aubert B, Quilici S. 1984. Biological control of the African and Asian citrus psyllids (Homoptera: Psylloidea), through eulophid and encyrtid parasites (Hymenoptera: Chalcidoidea) in Réunion Island, pp. 100-108. In Garnsey SM, Timmer LW, Dodds JA (editors), Proceedings, 9th Conference of the International Organization of Citrus Virologists (IOCV), 9–13 Nov. 1983, Argentina. University of California, Riverside.
Aubert B, Grisoni M, Villemin M, Rossolin G. 1996. A case study of huanglongbing (greening) control in Réunion. pp. 276-278 In da Graça JV, Moreno P, Yokomi RK (editors), Proceedings, 13th Conference of the International Organization of Citrus Virologists (IOCV). University of California, Riverside.
Bové JM. 2006. Huanglongbing: A destructive, newly-emerging, century-old disease of citrus. Journal of Plant Pathology 88: 7–37.
Chien CC. 1995. The role of parasitoids in the pest management of citrus psyllid. In Proceedings of Symposium of Research and Development of Citrus in Taiwan. pp. 245–261.
Chien CC, Chu YI. 1996. Biological control of citrus psyllid, Diaphorina citri. pp. 93–105. In Taiwan Biological Pest Control in Systems of Integrated Pest Management, Food and Fertilizer Technology Center for the Asian and Pacific Region. Taipei, Republic of China on Taiwan.
da Graça JV. 1991. Citrus greening disease. Annual Review of Phytopathology 29: 109–136.
Etienne J, Aubert B. 1980. Biological control of psyllid vectors of greening disease on Réunion Island. pp. 118–121. In Cavalan EC, Garnsey SM, Timmer LW (editors), In Proceedings of the 8th International Organization of Citrus Virologists International Organization of Citrus Virologists, Riverside, California.
Halbert SE. (May–June 1998). Entomology section: Citrus. Tri-ology. 37 (3) http://edocs.dlis.state.fl.us/fldocs/doacs/PlantIndustry/triology/3703.pdf (May 2021)
Halbert SE. 2005. The discovery of huanglongbing in Florida. In Proceedings of 2nd International Citrus Canker and Huanglongbing Research Workshop. Florida Citrus Mutual. Orlando, FL.
Halbert SE, Manjunath L. 2004. Asian citrus psyllid (Stenorrhynca: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Florida Entomologist 87: 330–353.
Hoy MA. 2003. Insect Molecular Genetics. Elsevier Science: Academic Press, San Diego, California. pp. 544.
Hoy MA. 2005. Classical biological control of citrus pests in Florida and the Caribbean: interconnections and sustainability, pp. 237-253. In Second International Symposium Biological Control of Arthropods, 12–16 September 2005, Davos, Switzerland.
Hoy MA, Nguyen R. 2000. Classical biological control of Asian citrus psylla. Citrus Industry 81(12): 48-50.
Jeyaprakash A, Hoy MA. 2000. Long PCR improves Wolbachia DNA amplification: WSP sequences found in 76% of sixty-three arthropod species. Insect Molecular Biology 9: 393–405.
Michaud JP. 2002. Biological control of Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae) in Florida: A preliminary report. Entomological News 113: 216–222.
Myartseva SN, Trjapitzin VA. 1978. Aphidencyrtus diaphorinae| sp.n. (Hymenoptera, Encyrtidae), a parasite reared from Diaphorina citri in Vietnam. JOURBOOK: Zoologicheskiy Zhurnal 57: 793–795.
Qureshi JA, Rogers ME, Hall DG, Stansly PA. 2009. Incidence of invasive Diaphorina citri (Hemiptera: Psyllidae) and its introduced parasitoid Tamarixia radiate (Hymenoptera: Eulophidae) in Florida citrus. Journal of Economic Entomology 102: 247–256.
Rohrig E, Shirk PD, Hall DG, Stansly PA. 2011. Larval Development of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae), an endoparasitoid of Diaphorina citri (Hemiptera: Psyllidae). Annals of the Entomological Society of America 104: 50–58.
Rohrig E, Hall DG, Qureshi JA, Stansly PA. 2012. Field release in Florida of Diaphorencyrtus aligarhensis (Hymenoptera: Encyrtidae) an endoparasitoid of Diaphorina citri (Homoptera: Psyllidae) from mainland China. Florida Entomologist 95: 479-481.
Ruggiero M, Gordon D, Bailly N, Kirk P, Nicolson D. 2011. The Catalogue of Life Taxonomic Classification, Edition 2, Part A. In Species 2000 & ITIS Catalogue of Life: 2011 Annual Checklist. Bisby FA, Roskov YR, Orrell TM, Nicolson D, Paglinawan LE, Bailly N, Kirk PM, Bourgoin T, Baillargeon G, Ouvrard D (eds). DVD; Species 2000: Reading, UK.
Shafee SA, Alam SM, Agarwal MM. 1975. Taxonomic survey of encyrtid parasites (Hymenoptera: Encyrtidae) in India. Aligarh Muslim University Publications (Zoological Series) of Indian Insect Types 10: 1–125.
Skelley LH, Hoy MA. 2004. A synchronous rearing method for the Asian citrus psyllid and its parasitoids in quarantine. Biological Control 29: 14–23.
Werren JH. 1997. Biology of Wolbachia. Annual Review of Entomology 42: 587–609.
Yang Y, Huang M, Andrew G, Beattie C, Xia Y, Ouyang G, Xiong J. 2006. Distribution, biology, ecology and control of the psyllid Diaphorina citri Kuwayama, a major pest of citrus: A status report for China. International Journal of Pest Management 52: 343–352.