Introduction
Wireworms are the larvae of click beetles. These larvae are smooth, slender and round in cross-section. Wireworms are important pests of various crops and occur in all seasons at variable densities (Seal et al. 1991; Seal et al. 1992c). Feeding damage is restricted to the seeds, seedlings and underground parts of the plants. Unlike foliage feeders, they are very difficult to detect due to their soil-dwelling habitat.
The Conoderus rudis (Brown) wireworm is a polyphagous soil insect. It is a major pest of various root crops. In Florida, fields planted to sweet potato, Ipomoea batatas (L.), had more wireworms than those planted to other crops. A fallow field with weeds provides a good habitat for C. ruids wireworm populations (Seal et al. 1994). Information on their biology is essential in controlling wireworms (Chalfant et al. 1991; Seal 1990; Seal et al. 1992a) and the previous infestation of a field is an important factor for the population abundance of this insect (Seal 1992b). However, initiation of infestation in a new field is by immigrant adults from the adjacent fields.
Distribution
Conoderus rudis is common in lands with a previous history of weed and grass in the region of its distribution (Seal 1992b). In the United States, C. rudis occurs in potato and corn fields in Georgia, South Carolina, North Carolina, Alabama, Louisiana, and Florida (Chalfant et al. 1990; Schalk et. 1986).
Description and Life Cycle
Adults
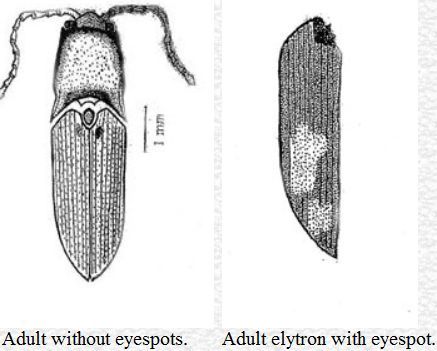
The adults are 5–8 mm in length. The body is deeply pitted and pale red in color. The head capsule is dorsally convex and broader (0.9–1.4 mm) than it is long (0.7–0.9 mm). The antenna is 11-segmented, with the first segment being the longest and the second segment the shortest. There is an 'eyespot' on each elytron that varies from light yellow to bright yellow color. Occasionally, the eyespot is absent. The adults are found on the surface of the soil hiding in the crevices around the stem of the host plants. They also remain hidden under leaves, grasses, and rotten logs during the day time. Adults are attracted by light at night. They do not damage roots, but feed on extrafloral secretions of various plants. The adult stage lasts for 21–38 days (mean, 25.50 days).

Credit: Dakshina R. Seal, UF/IFAS
Eggs
The adult lays eggs in the crevices or cracks on the soil surface near the root systems of host plants. The embryonic period lasts from five to ten days.
Larvae
The first instars are about 2.4 mm long. They are reddish brown with deep brown head capsules. The paired urogomphi (extension of the terminal segment) of the caudal notch, form a nearly closed V-shaped structure, and can be confused with other related species. Larvae stay close to the feeding host. The duration of first instars ranges from 26 to 32 days (mean, 29.8 days).
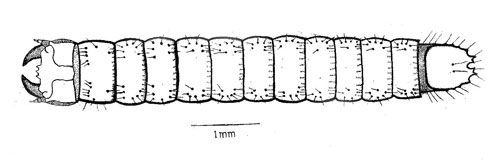
The second instars are 4.20 mm long, double the size of the first instar. They are reddish brown in color. The paired urogomphi of the ninth abdominal segment show a well separated V-shaped caudal notch which is almost similar to the third instar. The duration of the second instar ranges from 19 to 28 days (mean, 25.5 days).
The third instars average 8.34 mm in length. They are reddish brown in color with stout body. The third instars move vertically at 5–10 cm of the soil surface and horizontally at 5–20 cm around the feeding location. It has a distinctly separated, shallow V-shaped caudal notch. The duration of the third instar ranges from 15 to 25 days (mean, 20.4 days).
Prepupa and Pupa
The prepupal stage is characterized by a shortening in length, reduction in girth, and decrease in body activity. Before pupation, larvae move vertically upward to the surface of the soil. The prepupa is always formed inside a cell. The prepupal period lasts from 10 to 15 days (mean, 12.8 days). The pupal period lasts for seven to 13 days (mean, 8.02 days).
Hosts
Conoderus rudis feeds on roots of various vegetable and weed species. The larvae of C. rudis were collected from bean, cabbage, corn, cucumber, okra, squash, sweet potato, and tomato in south Florida (Seal, D.R., unpublished data). Further studies need to be conducted in various regions of the US to determine its host range.
Damage
Damage to the host crops is caused due to the feeding of C. rudis larvae. The first instars cause damage on the surface of the host roots and tubers by scraping the epidermal layer with mandibles. First instar feeding damage disappears over time with the growth of root tissues. The second instars actively feed on host roots, making shallow and narrow holes. These holes later heal due to the growth of root tissues. This damage is visible at harvest and known as 'healed holes'. The third instars are active at the beginning and make shallow and round feeding holes of 3–5 mm deep and 1–2 mm diameter on potato and other hosts. The feeding damage caused by the third instars at the early stage of root and tuber crop groth also heals over time and is known as healed holes, while the feeding holes at the late growth stage of roots and tubers are known as 'shallow holes.' Feeding damage of second and third instars causes patchy distribution of crops in a newly seeded field.
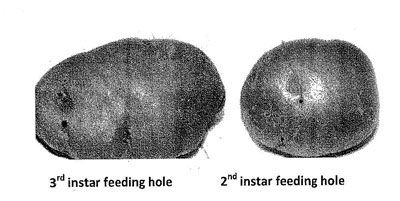
Economic Importance
Conoderus rudis larvae cause damage to seeds of various vegetable crops at germination and significantly reduce total stands in heavy infestations. In root and tuber crops, C. rudis feeding damage reduces marketable yield. Feeding damage also may serve allow entry for secondary pests and fungal pathogens. Crops known to be damaged by C. rudis include the following:
-
cucumber, Cucumis sativus
-
malanga, Xanthosoma caracu
-
peanut, Arachis hypogaea
-
potato, Solanum tuberosum
-
squash, Cucurbita pepo
-
sweet corn, Zea mays
-
sweet potato, Ipomoea batatas
(Cuthbert 1967)
Management
The use of proper cultural practices significantly reduces C. rudis populations. Cultivation of soil several times before planting exposes immature stages of C. rudis to birds and other predators. This practice also significantly reduces soil moisture resulting in desiccation of larvae and pupae. Keeping fields fallow (without weeds) for a season drastically reduces C. rudis population, as fallow fields containing grass support survival and reproduction of C. rudis. Flooding fields for a week reduces all stages of C. rudis. The previous history of the field is important in managing wireworms. Root and tuber crops should not be planted back to back into a previously infested field. To discontinue the C. rudis reproduction cycle, these crops should be rotated with fruiting and other vegetable crops. All raised beds from previous crops should be demolished during the preparation of the field for the next crop. Raised beds are a good habitat fo C. ruids and other wireworms. Placement of corn-wheat baits before planting or during crop season can be useful in attracting and killing wireworm larvae. Chemical control is not effective unless used at the time of their vertical movement behavior.
Selected References
Chalfant RB, Seal DR. 1991. Biology and management of wireworms on sweet potato. pp. 303-326. In Jansson RK, Raman KV [editors]. Westview, Boulder, CO: Sweet potato pest management: a global perspective.
Chalfant RB, Jansson RK, Seal DR, Schalk JM. 1990. "Ecology and management of sweet potato insects." Annual Review of Entomology 35: 157–180.
Cuthbert Jr FP. 1967. Insects affecting sweet potatoes. U.S.D.A. Handbook 329.
Schalk JM, Peterson JK, Jones A, Dukes PD, Walter Jr WM. 1986. "The anatomy of sweet potato periderm and its relationship to wireworm, Diabrotica, Systena resistance." Journal of Agricultural Entomology 3: 350–356.
Seal DR. 1990. The biology of wireworms affecting sweet potatoes in Georgia. Ph.D. dissertation, University of Georgia, Athens.
Seal DR, Chalfant RB, Hall MR. 1991. "Distribution and density of wireworms and the damage in relation to different cultivars of sweet potato." Proceedings of the Florida State Horticultural Society 104: 284–286.
Seal DR, Chalfant RB, Hall MR. 1992a. "Effectiveness of different seed baits and baiting methods for wireworms (Coleoptera: Elateridae) in sweet potato." Environmental Entomology 21: 957–963.
Seal DR, Chalfant RB, Hall MR. 1992b. "Effects of cultural practices and rotational crops on abundance of wireworms (Coleoptera: Elateridae) affecting sweet potato in Georgia." Environmental Entomology 21: 969–974.
Seal DR, McSorley R, Chalfant RB. 1992c. "Seasonal abundance and spatial distribution of wireworms (Coleoptera: Elateridae) in Georgia sweet potato fields." Journal of Economic Entomology 85: 1802–1808.
Seal DR, Chalfant RB. 1994. "Bionomics of Conoderus rudis (Coleoptera: Elateridae): Newly reported pest of sweet potato." Journal of Economic Entomology 87: 802–809.