Introduction
The wireworm Conoderus scissus Schaeffer is an important pest of root and tuber crops. It is also known as the peanut wireworm.
Distribution
Conoderus scissus is distributed in the coastal plain of southeastern United States (Hoffman 2007). It has been reported from Georgia (Schaeffer 1909; Van Dyke 1932; Fattig 1951), North and South Carolina (Kirk 1970), Mississippi (Lago and Testa 1989), and Florida (Peck and Thomas 1998).
Description
Adult: The adult is brown and is unique for its uniform color pattern that makes it easier to separate from other members of the genus. The average body length and width are 10.22 mm and 1.98 mm, respectively.

Credit: Dakshina R. Seal, UF/IFAS
Eggs: The eggs are creamy white in color and spindle shaped. The adult lays eggs in the crevices or cracks on the soil surface near the root systems of host plants.
Larvae: The first instar is creamy-white. It is 2.88–4.61 mm (avg. 3.67 mm) in length. The prothorax is the widest part of the body. The length of the ninth abdominal segment is greater than other body regions and also greater than the width of the same. The paired urogomphi of the dorsal plate of the ninth abdominal segment show a clear, open 'V' shaped caudal notch. The depth of the caudal notch averages 0.07 mm.
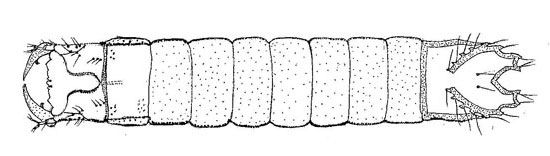
The last instar is whitish in color. It is 13–18 mm (avg. 15.55 mm) in length. The width of each body region exceeds the corresponding length except for the ninth abdominal segment. Various characters of full grown larvae are important to identify wireworm species.
Identifying Characteristics of Mature Larva
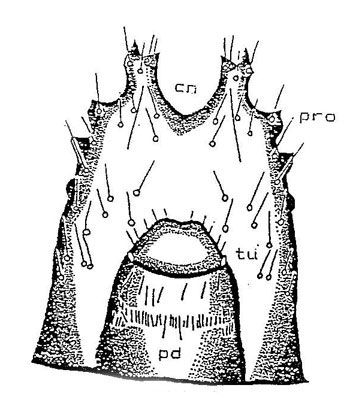
Frons: The shape of the frontal suture is distinct and is rounded posteriorly against the post occipital sulcus. Anteriorly, there are two sets of two setae inside the frontal suture. Above these sets there is a line of four other setae.
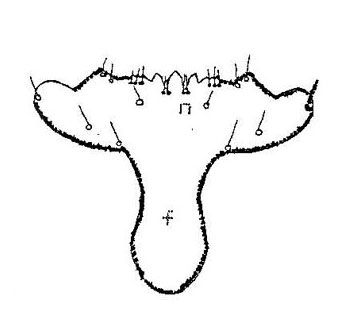
Gena: On the parietal side of the frons are three setae on the gena near the post occipital sulcus. Three more setae occur on the upper margin of each gena. On the outer margin of the gena, four setae are arranged in a line.
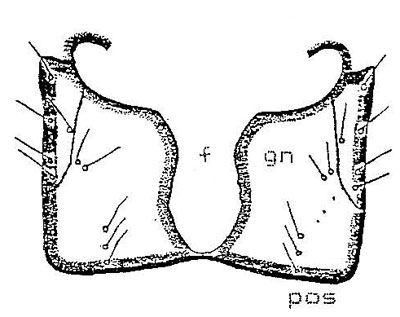
Ninth abdominal segment: 1) Dorsal surface. The dorsal plate of the ninth abdominal segment is provided laterally with six prominent protuberances on each side of the urogomphi, each of which is provided with a seta. There is one pair of setae separated by 0.21 mm on the dorsal surface 0.34–0.47 mm (avg. of 21 larvae, 0.41 mm) behind the groove of paired urogomphi. The paired urogomphi of the dorsal plate of the ninth abdominal segment show a clear, open 'V' shaped caudal notch.
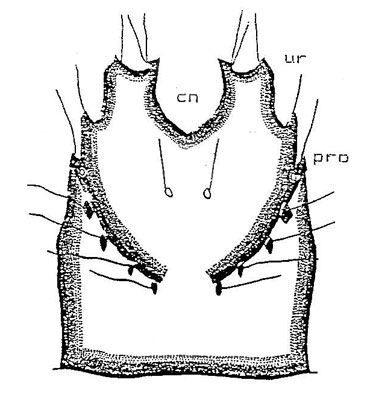
2) Ventral surface. The tip of the 10th abdominal segment (pseudopod) is eversible and has a tubercle on each side of the base. The pseudopod is covered with three rows of setae, one row around the tip of the pseudopod and the other two rows arranged cephlad.
Life Cycle and Biology
Conoderus scissus is more abundant in sandy soil than in muddy and rocky soil. Adults are active at night and can be caught in light traps. The life cycle lasts from two to three years with seven to 10 instars (Seal 1990; Chalfant and Seal 1991). In Florida, emergence of adults was recorded from March to September. All stages diapause after September and continue until March. Development of all diapausing stages (egg, larva, pupa, and adult) take place from March to early October.
Hosts
Conoderus scissus is polyphagous in nature (Kosmachevskii 1959; Guryeva 1969; Seal 1990). Larvae, commonly known as wireworms, feed on plants in the families Compositae, Cruciferae, Cucurbitaceae, Gramineae, Lilliaceae, Papilionaceae, Rosaceae, Solanaceae, Umbelliferae, and several others (Kosmachevskii 1959). Due to this polyphagous feeding habit, once a field is infested wireworm populations persist in the field even after the destruction of preferred hosts.
Economic Importance
Conoderus scissus is the most abundant wireworm species found in sweet potato fields. Its preference for other crops is followed by peanut, cowpea, and corn.
Management
Cultural practices are effective way of managing C. scissus. Knowing the previous history of a field is important in avoiding wireworm damage to planted crops. In a sand-rich soil, planting root crop and tuber crop back-to-back may increase C. scissus populations and feeding damage on the host crops. Fallowing land with or without weeds helps to reduce C.scissus populations. Disking fields deep provides adequate reduction of C.scissus resident populations. Flooding after disking increases mortality of larval and pupal stages. Attractive baits can be used to attract larvae and kill them. Corn-wheat mixture baits (50:50), which at germination attracts C. scissus larvae, can be buried at a depth of 10 cm. below the soil surface.
Selected References
Chalfant RB, Seal DR. 1991. Biology and management of wireworms on sweet potatoes. In Jansson RK, Raman KV (editors). Westview Press, Boulder, CO: Sweet Potato Pest Management A Global Perspective.
Fattig PW. 1951. "The elateridae or click beetles of Georgia." Emory University Museum Bulletin 10: 1–25.
Guryeva YL. 1969. "Some trends in the evolution of click beetles (Coleoptera: Elateridae)." Entomological Review 48: 154–159.
Hoffman RL. 2007. "The distribution of Conoderus scissus (Schaeffer) with notes on some taxonomic characters (Coleoptera: Elateridae:Agrypninae)." Banisteria 30: 32–34.
Kirk VM. 1970. "A list of the beetles of South Carolina Part 2 - Mountain, Piedmont, and Southern Coastal Plain." South Carolina Experimental Station Technical Bulletin 1038.
Kosmachevskii AS. 1959. "Biology of Agriotes litigiosus var tauricus Heyd. and Agriotes sputator L. (Coleoptera: Elateridae)." Entomological Review 38: 663–672.
Lago PK, Testa S. 1989. "The aquatic and semiaquatic Hemiptera and Coleoptera of Point Clear Island, Hancock County, Mississippi." Journal of the Mississippi Academy of Sciences 34: 33–38.
Peck SB, Thomas MC. 1998. "A Distributional Checklist of the Beetles (Coleoptera) of Florida." Gainesville, FL: Arthropods of Florida and Neighboring Land Areas, Floriuda Department of Agriculture and Consumer Services.
Schaeffer C. 1909. New Coleoptera chiefly from Arizona. Science Bulletin of the Museum, Brooklyn Institute of Arts and Sciences 1: 375–386.
Seal DR. 1990. Biology and Management of Wireworm (Coleoptera: Elateridae) Affecting Sweetpotato in Georgia. Ph.D. dissertation, University of Georgia, Athens, GA, USA.
Van Dyke EC. 1932. "Miscellaneous studies in the Elateridae and related families of Coleoptera." Proceedings of the California Academy of Science 20: 291–405.