Introduction
The tropical bont tick, Amblyomma variegatum Fabricius, is a three-host tick that originated in Africa (Yonow 1995). It has since spread to several countries, including the Caribbean islands, where it is known as the Senegalese tick (CaribVet 2011b) and the Antigua gold tick (Pegram et al. 2004). The name 'Senegalese tick' came about because of the suspected introduction of the tick from cattle imports from Senegal to the Caribbean (Barré et al. 1995). They are vividly colored and decorated ticks, especially the males (CaribVet 2011b; Merck 2011).
The tropical bont tick has had a huge effect on the livestock industry, primarily through its transmission of heartwater disease, Ehrlichia ruminantium (formerly Cowdria ruminantium) (Allan et al. 1998; CaribVet 2011b; OIE 2009; Parola et al. 1999) and their association with dermatophilosis, Dermatophilus congolensis (Allan et al. 1998; Barré and Garris 1990; CaribVet 2011b; Merck 2011). The tropical bont tick has also been implicated as a vector or potential vector for several diseases that include Crimean-Congo hemorrhagic fever virus, Dugbe virus, yellow fever virus, Rickettsia africae (African tick bite fever) and Jos virus (Merck 2011). In the Caribbean, only heartwater disease and dermatophilosis have yet been detected in the hosts and have demonstrated clinical symptoms. The testing of ticks and seropositive blood tests of cattle have led to the conclusion that African tick bite fever is widespread in the islands, but there have been few positive human case reports (Kelly et al. 2010; NTHNC 2008). There is a low incidence of documented reports of infection by other diseases in association with the tropical bont tick, and they occur primarily in central Africa (Merck 2011).
Distribution
The tropical bont tick originated in Africa (Allan et al. 1998; Barré and Garris 1990; Merck 2011). It has a wide range of distribution, as it tolerates a range of environments from dry savannahs to more humid regions, such as forests (Barré et al. 1995). The tick also has been reported in Madagascar, Zanzibar, the Comoros, the Mascarene Islands, the Caribbean, and southern Arabia (Barré et al. 1995; Merck 2011). Since its known introduction to the Caribbean in 1895, and possibly as early as 1864 (Pegram and Eddy 2002; Pegram et al. 2004), the tick is now considered endemic on Guadeloupe, Antigua, and Marie Galante (Barré and Garris 1990). The tropical bont tick has also been found in Puerto Rico and many other Caribbean islands (Allan et al. 1998). Currently, it has not been identified in the United States, but there is a genuine concern for its introduction because of its increase in rate of spread through the Caribbean over the past few years, as well as the increases in exotic and domestic animal importation/exportation (Jongejan 1992; Pegram and Eddy 2002).
The dispersal of tropical bont tick is associated with the migration patterns of the cattle egret, Bubulcus ibis (OIE 2009; Barré et al. 1995; Pegram and Eddy 2002). The birds transport the larvae and the nymphs of the tick (Deem 1998). Because the non-feeding stages of the life cycle occur within the environment and not on the host, the transport of infested vegetation and litter is a potential source for dispersion as well (Alderink and McCauley 1988; Barré et al. 1995).

Credit: Joy Viola, Northeastern University; bugwood.org
Description
The tropical bont tick is relatively large and has a bright coloration that makes it easily identifiable (CaribVet 2001b; Merck 2011; Pegram et al. 2004). The sometimes bright, yellow-gold coloration that is seen in the males has led to the common name, Antigua gold tick (CFSPH 2006, Pegram et al. 2004). Females are usually brown and when fully engorged can be the size of a "nutmeg" (approximately 2 to 3 centimeters long) (CFSPH 2006). As a member of the family Ixodidae, the tropical bont tick is considered a hard tick and has a scutum (Georgi and Georgi 1990). In females the scutum is smaller with a wide posterior angle and straight sides (Walker et al. 2007). Due to the scutum being smaller, it only provides partial coverage of the dorsal surface, which, as feeding or engorgement commences, covers a progressively smaller percentage of her body (Georgi and Georgi 1990). "The posterior lips of the female genital aperture forms a wide shaped 'U'" (Walker et al. 2007). In general, tropical bont ticks also have long and thick mouthparts that allow them to become firmly embedded in their hosts (Georgi and Georgi 1990). The subsequent damage from the mouthparts predisposes the host to infection from various diseases such as dermatophilosis (CaribVet 2011a; Merck 2011). Some other features present in this tick are long palps that have a long, narrow, second segment, the presence of distinctly convex eyes, and well developed festoons or bulges that appear at the posterior edge of the abdomen when not feeding (CFSPH 2006; Walker et al. 2007). There is no enamel on the festoons as may be seen with other Amblyomma spp. (Walker et al. 2007). As tick identification can be difficult, any suspected Amblyomma variegatum should be sent to state or federal agricultural services, or your local county Cooperative Extension Service.

Credit: Alan Walker, University of Edinburgh

Credit: Richard Matthews and Alan Walker, University of Edinburgh
Life Cycle and Biology
The tropical bont tick is a three-host tick, where each life stage (larva, nymph, and adult) completes a blood meal on a particular host before dropping off and molting (Barré et al.1995; CaribVet 2011b). The hosts for each stage may or may not be the same species (Barré et al 1995). The ticks tend to congregate in hard-to-reach areas such as the underbelly, the dewlap, genitalia, and under the tail near the anus (Barré and Garris 1990; CaribVet 2011b; Merck 2011).

Credit: CIRAD, CaribVet
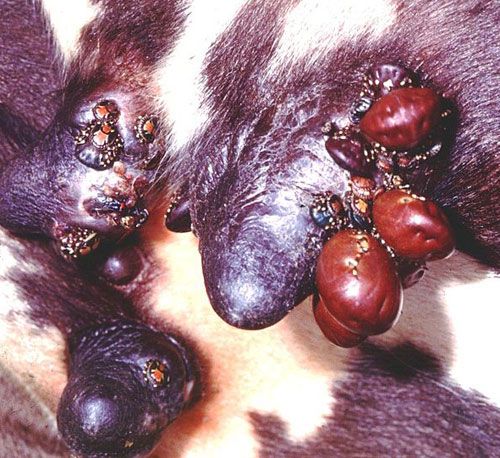
Credit: Alan Walker, University of Edinburgh
The females generally do not attach to the hosts until males have been present for at least three days (Barré and Garris 1990). The males secrete pheromones to attract females for sexual reproduction (Allan et al. 1998; Popham et al. 1996). Once mated, the females attach themselves to the host where they remain for approximately two weeks (Barré and Garris 1990). The females turn from a brown-black color to an orange color just prior to detachment from the host (Popham et al. 1996). Each female can produce up to 30,000 eggs (Barré et al. 1995; CaribVet 2011b). There can be three generations produced every two years (Popham et al. 1996).
Hatching time depends on temperature and ranges from 50 to 100 days, with fewer days required in warmer weather (Pegram and Banda 1990). After hatching, the larvae have been shown to survive in the environment for a couple of months during cooler periods (Pegram and Banda 1990) and will congregate on vegetation in search of hosts (Barré et al. 1995). Larval and nymphal periods also vary with temperature and molt usually occurs on an average of 45 days (Pegram and Banda 1990).
Feeding times vary based on seasons with the adults feeding during the rainy season and the immature stages feeding during the dry (Merck 2011). After the final molt into the adult stage, A. variegatum remains dormant for four weeks before any host seeking occurs (Pegram and Banda 1990).
Overall, very little time is spent on the host feeding, a maximum of 15 days for each stage of development (Popham et al. 1996; Yonow 1995). Most of the life cycle occurs on the ground and in the vegetation, which makes the tick sensitive to extremes in temperature. As a result, it inhabits the warmer subtropical and tropical regions (Yonow 1995).
Hosts
The adult ticks are found on various domesticated species such as camels, cattle, goats, sheep, and even dogs (Deem 1998; Merck 2011). The ticks also are found on various species of wildlife throughout the distribution range, but the adults are generally found on the larger mammals (Barré et al.1995; Yonow 1995).
The immature stages may also be present on ruminants (Barré et al. 1995), but generally are present in greater numbers on smaller mammals and birds, such as the mongoose and cattle egret (Barré and Garris 1990; OIE 2011; Pegram and Eddy 2002; Pegram et al. 2004).
Medical and Veterinary Significance
The tropical bont tick is considered one of the most detrimental of the tick species present in Africa and now the Caribbean (CaribVet 2011b; Stachurksi and Lancelot 2006). It can result in severe economic losses due to hide damage, milk production reduction, and death of livestock (Norval et al. 1992; Walker 1996). The two primary diseases of concern that are associated with the tropical bont tick are dermatophilosis and heartwater (CaribVet 2011a; CaribVet 2011b; Merck 2011).
The tick has been associated with dermatophilosis caused by gram positive bacteria, Dermatophilus congolensis (CaribVet 2011a). The disease is not actually transmitted by the tick but from damage that results from wounds caused by the large mouthparts and the immunosuppression (CaribVet 2011a, Walker 1996) that occurs secondary to feeding, which predisposes entry of the bacteria into the skin (CaribVet 2011a; Merck 2011a). Dermatophilosis results in a loss of milk production, poor quality and often unusable hides, weight loss, and sometimes death, which is more common in acute forms (CaribVet 2011a).

Credit: © FAO/Maria Einarsson
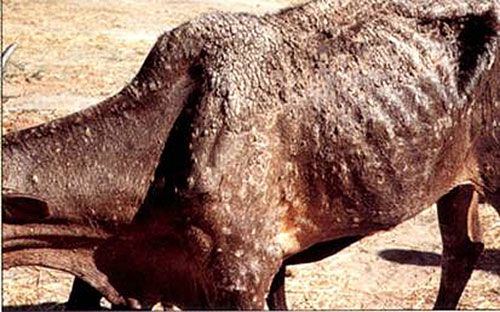
Credit: CIRAD, CaribVet
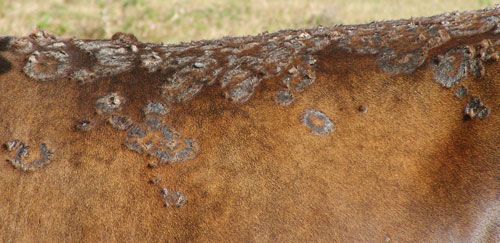
Credit: CIRAD, CaribVet
Transmission of diseases, such as heartwater, requires the larvae or nymphs to feed on infected or reservoir hosts (CaribVet 2011b). For heartwater disease there is no trans-ovarian transmission, or transmission of the disease from the adult tropical bont tick to her offspring (CaribVet 2011b). If heartwater were to be introduced into the US, the disease could potentially be spread by existing species of Amblyomma currently occurring in the US (Barré et al. 1987). Of the North American species, the greatest concern is A. maculatum Koch, the Gulf coast tick, as it has been shown, experimentally, to have a high potential for transmission and it is widely distributed in the US (OIE 2009). The other species of concern are A. cajennense (Fabricius), the cayenne tick, and A. dissimile Koch (Jongejan 1992; OIE 2009). Heartwater, Ehrlichia ruminantium, affects ruminants, cattle in particular, and can have a death rate of 80% in susceptible animals (Barré and Garris 1990). Clinical signs range from no symptoms to elevated temperatures, respiratory distress, and death (CaribVet 2011a; Merck 2011). As the name suggests, fluid develops around the heart and lungs (CaribVet 2011a).

Credit: James Castner, University of Florida
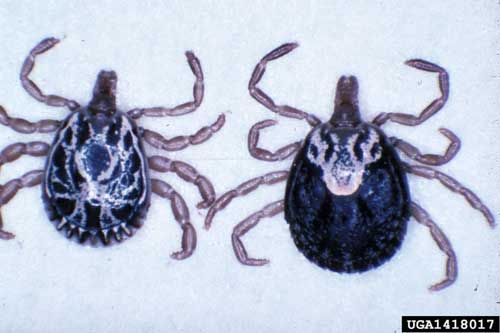
Credit: Mat Pound, USDA Agricultural Research Service, Bugwood.org

Credit: Carmen Guzman-Cornejo, Instituto de Biologia, UNAM
Other animal diseases associated with the tropical bont tick include Crimean-Congo hemorrhagic fever virus (which may also affect humans), Dugbe virus, and Jos virus (Merck 2011).
A disease of human health concern transmitted by the tropical bont tick is African tick-bite fever, caused by Rickettsia africae, which results in fevers, headaches, and swollen lymph nodes (Parola et al. 1999). The distribution of African tick-bite fever is considered widespread in the Caribbean, but there have been only a few positive human cases reported (Kelly et al. 2010; NTHNC 2008). Although the tick is not considered a vector of yellow fever, it may serve as a potential concern for human infection, as well as a reservoir for the disease as the virus has been isolated from it (CDC 2001; Merck 2011).
Besides disease transmission, the tropical bont tick can cause significant blood loss in its hosts (Ndumu et al. 1999). Each female can consume up to 20 milliliters of blood before dropping off the host to lay her eggs (Walker 1996). In cases where infestation is severe (CaribVet 2011b), there can also be a loss of appetite and weight that predisposes the host to other diseases (Walker 1996). It is not unheard of for there to be several hundred ticks present on a single host (Popham et al. 1996).
Management
Between hosts, the ticks undergo life cycle phases such as molting for the larvae and nymphs, and egg-laying for the adult females (Barré et al. 1995). These stages occur on the ground or on vegetation (Barré et al. 1995). All stages have the capability of surviving host-less in the environment for months to years depending on environmental conditions (Barré and Garris 1990; Pegram et al. 2004). Due to the tick's ability to survive in the environment for extended periods, it is recommended that livestock are not reintroduced into pasture that had been vacated in an attempt at eradication for a period of not less than 46 months (Barré and Garris 1990).
Primary control of tropical bont tick is through the use of acaricides (Norval et al. 1992). Use of acaricides should coincide with the on-host phases of the tick and focus on areas of aggregation (Barré and Garris 1990). Some work has been done using a combination of pheromone and acaricides to help reduce the costs associated with the chemical as well as improve kill efficacy (Allan et al. 1998, Norval et al.1992). Footbaths containing acaricides have been used with some success, as the ticks often will attach between the hooves (Stachurski and Lancelot 2006). The use of Neem seed oil can be effective but is very dependent on the concentration and contact time (Ndumu et al. 1999).
In Africa, birds are the primary means of biological control of ticks, but have the potential to cause additional skin damage as they peck at the host's skin (Samish 2006). In regions such as the Caribbean, birds are not as successful at controlling the ticks (Popham et al. 1996). An attempt made using a parasitic wasp, Ixodiphagus sp., was shown to reduce the host nymph populations for approximately two years but a sustained and effective population of the wasp could not be maintained (Samish 2006). Other methods of biological control such as nematodes, bacteria, and fungi have also not been very successful to date (Samish 2006).
Eradication campaigns are currently under way [OR in progress] in the Caribbean to end the economic losses and perhaps prevent further spread of A. variegatum (Barré and Garris 1990; Barré et al. 1995; Pegram and Eddy 2002).
Selected References
Alderink FJ, McCauley EH. 1988. The probability of the spread of Amblyomma variegatum in the Caribbean. Preventive Veterinary Medicine 6: 265–294.
Allan SA, Barré N, Sonenshine DE, Burridge, MJ. 1998. Efficacy of tags impregnated with pheromone and acaricide for control of Amblyomma variegatum. Medical and Veterinary Entomology 12: 141–150.
Barré N, Uilenberg G, Morel PC, Camus E. 1987. Danger of introducing heartwater onto the American mainland: potential role of indigenous and exotic Amblyomma ticks. Onderstepoort Journal of Veterinary Research 54: 405–417.
Barré N, Garris GL. 1990. Biology and ecology of Amblyomma variegatum (Acari: Ixodidae) in the Caribbean: implications for a regional eradication program. Journal of Agricultural Entomology 7: 1–9.
Barré N, Garris G, Camus E. 1995. Propagation of the tick Amblyomma variegatum in the Caribbean. Scientific and Technical Review of the Office International des Epizooties 14: 841–855.
(CaribVet). (January 2011a). Dermatophilosis. Caribbean Animal Health Network. http://www.caribvet.net/en/monograph-0 (20 February 2012).
(CaribVet). (January 2011b). Heartwater. Caribbean Animal Health Network. http://www.caribvet.net/en/diseases/heartwater/monograph (13 August 2012).
Center for Disease Control and Prevention (CDC). (2001). Yellow fever surveillance-Africa. Morbidity and Mortality Weekly Report. http://www.cdc.gov/mmwr/preview/mmwrhtml/00001149.htm (7 July 2017).
Center for Food Security and Public Health (CFSPH). (2006). Amblyomma variegatum. International Veterinary Information Service. http://www.ivis.org/advances/Disease_Factsheets/amblyomma_variegatum.pdf (20 February 2012).
Deem SL. 1998. A review of heartwater and the threat of introduction of Cowdria ruminantium and Amblyomma spp. ticks to the American mainland. Journal of Zoo and Wildlife Medicine 29: 109–133.
Georgi JR, Georgi ME. 1990. Arthropods: family Ixodidae, pp. 1–76. In Parasitology for Veterinarians, 5th edition. W. B. Saunders Co, Philadelphia, PA. pp 412.
Jongejan F. 1992. Experimental transmission of Cowdria ruminantium (Rickettsiales) by the American reptile tick Amblyomma dissimile Koch, 1844. Experimental and Applied Acarology 15: 117–121.
Kelly P, H Lucas, L Beati, C Yowell, S Mahan, J Dame. 2010. Rickettsia africae in Amblyomma variegatum and domestic ruminants on eight Caribbean islands. Journal of Parasitology 96: 1086–1088.
(Merck) (2011). Important Ixodid ticks: Amblyomma spp. The Merck Veterinary Manual. http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/72107.htm (15 February 2012).
National Travel Health and Network Centre (NTHNC). 2008. Travel Health Information Sheets: Rickettsial Diseases. http://www.nathnac.org/pro/factsheets/rick.htm (15 May 2012).
Ndumu PA, George BD, Choudhury MK. 1999. Toxicity of Neem seed oil (Azidiracta indica) against the larvae of Amblyomma variegatum, a three-host tick in cattle. Phytotherapy Research 13: 532–534.
Norval RAI, Peter T, Sonenshine DE, Burridge ME. 1992. Responses of the ticks Amblyomma hebraeum and A. variegatum to known or potential components of the aggregation-attachment pheromone. III. Aggregation. Experimental and Applied Acarology 16: 237–245.
OIE (2009). Heartwater – Cowdriosis. World Organization for Animal Health. http://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/2008/pdf/2.01.06_HEARTWATER.pdf (7 July 2017).
Parola P, Vestris G, Martinez D, Brochier B, Roux V, Raoult D. 1999. Tick-borne rickettiosis in Guadeloupe, the French West Indies: isolation of Rickettsia africae from Amblyomma variegatum ticks and serosurvery in humans, cattle, and goats. American Journal of Tropical Medicine and Hygiene 60: 888–893.
Pegram RG, Banda DS. 1990. Ecology and phenology of cattle ticks in Zambia: development and survival of free-living stages. Experimental and Applied Acarology 8: 291–301.
Pegram RG, Eddy C. 2002. Progress towards the eradication of Amblyomma variegatum from the Caribbean. Experimental and Applied Acarology 28: 273–281.
Pegram R, Indar L, Eddi C, George J. 2004. The Caribbean Amblyomma program: some ecologic factors affecting its success. Annals of the New York Academy of Sciences 1026: 302–311.
Popham TE, Garris GI, Barré N. 1996. Development of a computer model of the population dynamics of Amblyomma variegatum and simulations of eradication strategies for use in the Caribbean. Annals of the New York Academy of Sciences 791: 452–465.
Samish M. 2006. Biocontol of ticks. Annals of the New York Academy of Sciences 916: 172–178. DOI: 10.1111/j.1749-6632.2000.tb05287.x
Stachurski F, Lancelot R. 2006. Footbath acaricide treatment to control cattle infestation by the tick Amblyomma variegatum. Medical and Veterinary Entomology 20: 402–412.
Walker AR. 1996. Amblyomma tick feeding in relation to host health. Tropical Animal Health and Production 28: 26S–28S.
Walker AR, Bouattour A, Camicas JL, Estrada-Peña A, Horak IG, Latif AA, Pegram KG, Preston PM. 2007. Ticks of Domestic Animals in Africa: A Guide to Identification of Species. Bioscience Reports. 221 pp.
Yonow T. 1995. The life-cycle of Amblyomma variegatum (Acari: Ixodidae): a literature synthesis with a view to modeling. International Journal for Parasitology 9: 1023–1060.