Introduction
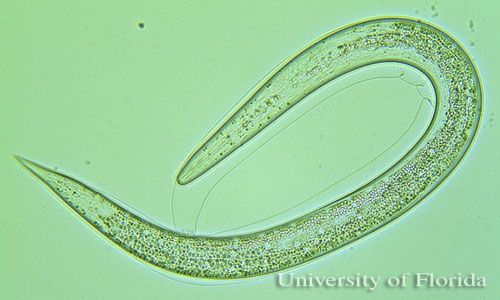
Entomopathogenic nematodes are soft bodied, non-segmented roundworms that are obligate or sometimes facultative parasites of insects. Entomopathogenic nematodes occur naturally in soil environments and locate their host in response to carbon dioxide, vibration, and other chemical cues (Kaya and Gaugler 1993). Species in two families (Heterorhabditidae and Steinernematidae) have been effectively used as biological insecticides in pest management programs (Grewal et al. 2005). Entomopathogenic nematodes fit nicely into integrated pest management, or IPM, programs because they are considered nontoxic to humans, relatively specific to their target pest(s), and can be applied with standard pesticide equipment (Shapiro-Ilan et al. 2006). Entomopathogenic nematodes have been exempted from the US Environmental Protection Agency (EPA) pesticide registration. There is no need for personal protective equipment and re-entry restrictions. Insect resistance problems are unlikely.
Credit: James Kerrigan, UF/IFAS

Credit: Robin Bedding, CSIRO
Life Cycle
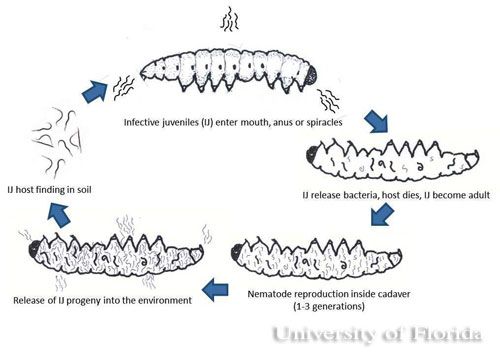
The infective juvenile stage (IJ) is the only free living stage of entomopathogenic nematodes. The juvenile stage penetrates the host insect via the spiracles, mouth, anus, or in some species through intersegmental membranes of the cuticle, and then enters into the hemocoel (Bedding and Molyneux 1982). Both Heterorhabditis and Steinernema are mutualistically associated with bacteria of the genera Photorhabdus and Xenorhabdus, respectively (Ferreira and Malan 2014). The juvenile stage release cells of their symbiotic bacteria from their intestines into the hemocoel. The bacteria multiply in the insect hemolymph, and the infected host usually dies within 24 to 48 hours. After the death of the host, nematodes continue to feed on the host tissue, mature, and reproduce. The progeny nematodes develop through four juvenile stages to the adult. Depending on the available resources, one or more generations may occur within the host cadaver, and a large number of infective juveniles are eventually released into environment to infect other hosts and continue their life cycle (Kaya and Gaugler 1993).
Credit: Steven Arthurs, UF/IFAS
Reproduction differs in heterorhabditid and steinernematid nematodes. Infective juveniles of heterorhabditid nematodes become hermaphroditic adults, but individuals of the next generation produce both male and females whereas in steinernematid nematodes all generations are produced by males and females, (gonochorisism) (Grewal et al. 2005). The insect cadaver becomes red if the insects are killed by heterorhabditids and brown or tan if killed by steinernematids (Kaya and Gaugler 1993). The color of the host body is indicative of the pigments produced by the monoculture of mutualistic bacteria growing in the hosts.
Searching Behavior
Entomopathogenic nematodes use two search strategies: ambushers or cruisers (Grewal et al. 1994a). Ambushers such as S. carpocapsae have an energy-conserving approach and lie in wait to attack mobile insects (nictitating) in the upper soil. Cruisers like S. glaseri and H. bacteriophora are highly active and generally subterranean, moving significant distances using volatile cues and other methods to find their host underground. Therefore, they are effective against less mobile pests such as white grubs (Scarab beetles). Some nematode species, such as S. feltiae and S. riobrave, use an intermediate foraging strategy (combination of ambush and cruiser type) to find their host.
Production and Formulation
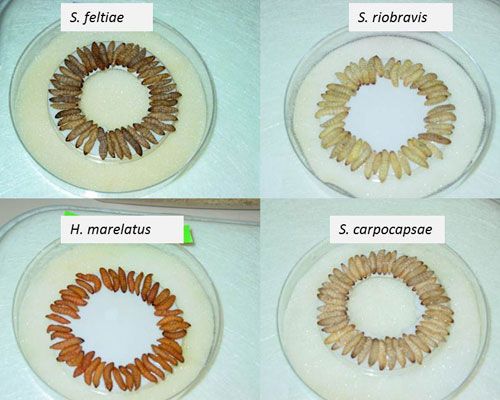
Entomopathogenic nematodes are currently produced by different methods either in vivo or in vitro (solid and liquid culture) (Shapiro-Ilan et al. 2012). In vivo production is a simple process of culturing a specific entomopathogenic nematode in live insect hosts, which requires minimal technology and involves the use of a surrogate host (typically larvae of wax moth (Galleria mellonella), trays and shelves. In vivo production uses a White trap (White 1927), which takes advantage of the juvenile stage's natural migration away from the host-cadaver. However, this method is not cost effective for scaled-up productions and may be only ideal for small markets or laboratory studies (Shapiro-Ilan et al. 2002). In vitro culturing of entomopathogenic nematodes is based on introducing nematodes to a pure culture of their symbiont in a nutritive medium. Significant improvements in in vitro culture utilizing large fermenters are used to produce large quantities of entomopathogenic nematodes for commercial use. Nematodes can be stored and formulated in different ways including the use of polyurethane sponges, water-dispersible granules, vermiculite, alginate gels and baits. Formulated entomopathogenic nematodes can be stored for 2 to 5 months depending on the nematode species and storage media and conditions. Unlike other microbial control agents (fungi, bacteria, and viruses) entomopathogenic nematodes do not have a fully dormant resting stage, and they will use their limited energy during storage. The quality of the nematode product can be determined by nematode virulence and viability assays, age, and the ratio of viable to non-viable nematodes (Grewal et al. 2005).
Credit: Heather Headrick, USDA

Credit: Lawrence Lacey, USDA
Handling and Effectiveness
Unsatisfactory results of entomopathogenic nematodes as pest control agents are caused by improper handling, transport, and storage (Shapiro-Ilan et al. 2002). Entomopathogenic nematodes are living organisms, and both biotic and abiotic factors can be detrimental during applications. Entomopathogenic nematodes work best in sandy soil with a pH between 4 and 8. Entomopathogenic nematodes are susceptible to freezing, hot temperatures, desiccation, and UV light. More heat tolerant species include S. ribrave, S. glaseri, and H. indica, while S. feltiae, H. megidis and H. marelatus are adapted to cooler temperatures (Grewal et al. 1994b). The nematode efficacy can be enhanced by matching the most appropriate species to the target pests, using the correct rate of a viable nematode product, keeping the treated area wet for at least 8 hours post application and applying during early morning or evening hours to minimize UV exposure and drying conditions. It is also important to inspect entomopathogenic nematodes after receiving them and prior to application to ensure that they are viable (sinusoidal movement of healthy juvenile stages can be observed with a 20 X hand lens or microscope).
Application Considerations
Entomopathogenic nematodes can be applied with most horticultural equipment, including pressurized sprayers, mist blowers, and electrostatic sprayers. The application equipment chosen will depend on the cropping system. In general large diameter nozzles (orifices) and high volumes (up to 400 gallons per acre) are recommended. Filters, screens, and swirl plates should be removed from spray equipment lines to prevent them from becoming clogged with infective juveniles. It is also important to ensure adequate agitation during application because entomopathogenic nematodes settle quickly in suspension. High pressures (> 300 psi) should also be avoided, and entomopathogenic nematodes can be kept cool by adding ice packs to the spray suspension. Aquarium bubbles can be used to provide aeration if nematodes are not used immediately. Studies have shown that entomopathogenic nematodes are compatible with many (but not all) insecticides, fungicides, and herbicides. Fresh manure or high rates of chemical fertilizers (e.g., urea) can be detrimental to entomopathogenic nematode's persistence and efficacy. Substantial progress has been made in recent years in developing entomopathogenic nematode formulations, particularly for aboveground applications, e.g., mixing entomopathogenic nematodes with particular surfactants and water dispersable polymers (Shapiro-Ilan et al. 2010).

Credit: Lawrence Lacey, USDA
Selected References
Bedding R, Molyneux A. 1982. "Penetration of insect cuticle by infective juveniles of Heterorhabditis spp. (Heterorhabditidae: Nematoda)." Nematologica 28: 354–359.
Ferreira T, Malan AP. 2014. "Xenorhabdus and Photorhabdus, bacterial symbionts of the entomopathogenic nematodes Steinernema and Heterorhabditis and their in vitro liquid mass culture: a review." African Entomology 22: 1–14.
Grewal P, Lewis E, Gaugler R, Campbell J. 1994a. "Host finding behaviour as a predictor of foraging strategy in entomopathogenic nematodes." Parasitology 108: 207–215.
Grewal PS, Selvan S, Gaugler R. 1994b. "Thermal adaptation of entomopathogenic nematodes-niche breadth for infection, establishment and reproduction." Journal of Thermal Biology 19: 245–253.
Grewal PS, Ehlers R-U, Shapiro-Ilan DI. 2005. Nematodes as Biocontrol Agents. New York, NY: CABI.
Kaya HK, Gaugler R. 1993. "Entomopathogenic nematodes." Annual Review of Entomology 38: 181–206.
Shapiro-Ilan DI, Han R, Dolinksi C. 2012. "Entomopathogenic nematode production and application technology." Journal of Nematology 44: 206–217.
Shapiro-Ilan DI, Gouge DH, Koppenhofer AM. 2002. Factors affecting commercial success: case studies in cotton, turf and citrus. In: Gaugler R. (Ed.), Entomopathogenic Nematology. New York, NY: CABI.
Shapiro-Ilan DI, Gough DH, Piggott SJ, Patterson Fife J. 2006. "Application technology and environmental considerations for use of entomopathogenic nematodes in biological control." Biological Control 38: 124–133.
Shapiro-Ilan DI, Cottrell TE, Mizell RF, Horton DL, Behle B, Dunlap C. 2010. "Efficacy of Steinernema carpocapsae for control of the lesser peachtree borer, Synanthedon pictipes: Improved aboveground suppression with a novel gel application." Biological Control 54: 23–28.
White GF. 1927. "A method for obtaining infective nematode larvae from cultures." Science 66: 302–303.