Horn flies (Figure 1) are one of the livestock pests with the greatest impact on the health and productivity of cattle. Economic losses due to horn fly damage are estimated at $36 million annually in Florida alone. In the US annual losses total between $700 million and $1 billion, with up to $60 million spent on insecticidal control.

Credit: J. F. Butler, University of Florida
Horn fly damage is caused by blood feeding. The flies feed frequently and exclusively on blood, piercing the skin of cattle with their proboscis and taking around 20 small blood meals each day. Pain and irritation due to the constant presence of the flies and their bites causes defensive behavior in the cattle that prevents adequate food consumption and rest.
Biology
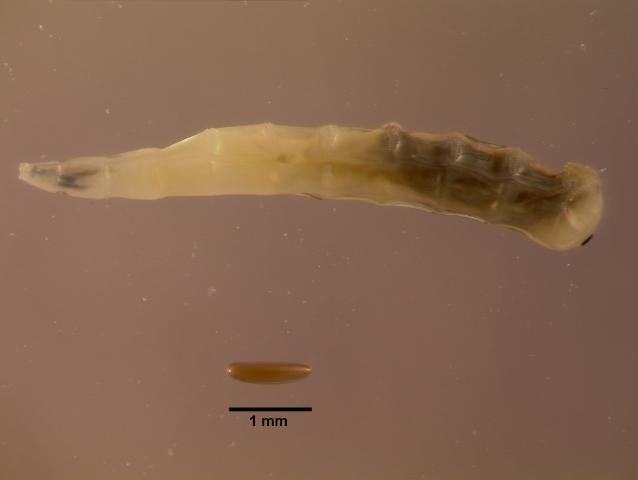
Horn flies (Figure 2) are small flies, about 3 to 5 mm long. The common house fly is usually 4–12 mm in length, over twice the size of the horn fly. Unlike the house fly, which has mouthparts suitable for soaking up liquid food, the horn fly has for piercing skin and sucking blood. Both males and females feed exclusively on blood. The adults are usually on the host, clustered on the withers, back, and sides, but will move to the belly during hot weather (Figure 3). Eggs are laid by the adult female in cattle manure that is very fresh, usually within 10 minutes of dropping. Horn fly eggs are oval, approximately 1.2 mm long and reddish-brown (Figure 4), making detection in manure difficult for monitoring.

Credit: D. Fitzpatrick, University of Florida.

Credit: P. E. Kaufman, University of Florida.
Credit: D. Fitzpatrick, University of Florida.
Within 18 hours the eggs hatch and the first stage larva or maggot emerges and starts to feed. At this stage the maggot is white and about 1.5 mm long with a pointed head. Development through three instars occurs in the manure over 3 to 5 days. The mature maggot, which is 6.5 to 7.5 mm long (Figure 4), pupates in the manure. The pupal stage is reddish-brown and 3 to 4 mm long (Figure 5). The adults emerge from the pupal stage after 3 to 5 days. Adult flies begin mating 3 to 5 days after they emerge, and the females start to lay eggs 3 to 8 days after emerging from the pupal stage. Development from egg to sexually mature adult takes from 10 to 14 days. Each female fly may lay up to 200 eggs, in batches of 14 to 17 eggs. Horn flies spend most of their time on the host, leaving only when it is necessary to relocate to a new animal or to lay eggs in freshly deposited manure. See EENY490 Horn Fly Haematobia irritans irritans for more information on horn fly biology.

Credit: E. N. I. Weeks, University of Florida.
In subtropical and temperate climates, the pest stage or the adult horn flies are present only in the warm months. The flies spend the winter as pupae. Initiation of the pupal stage is triggered by cooling temperatures and decreasing day length. The adults will not emerge until the conditions are warm enough in the spring. In Florida and other southeastern states, biting horn flies are present throughout the year, and although populations decline in the winter, they do not undergo a diapause (period of dormancy).
Damage
Horn flies are blood-feeding insects that take multiple blood meals from their cattle host daily. Defensive behavior, such as head-tossing and tail-switching to dislodge the flies, uses up the cattle's energy reserves and causes decreased growth and production. The irritation and blood loss caused by horn flies leads cattle to lose 0.3 to 0.5 lbs in weight per day, and reduces milk production in dairy animals. Although an individual horn fly only consumes 1.5 mg of blood per meal, horn flies take many meals per day, and large populations may cause substantial blood loss. Irritation of the skin due to high numbers of horn fly bites in small areas may cause open wounds, which can increase the risk of secondary infections. The bite lesions, particularly if infections develop, may lead to damaged hides and potentially reduce hide values. Horn flies are vectors of several pathogens, including a filarial nematode that causes skin disorders in cattle in the western and southwestern US and Canada. Horn flies have been shown to cause severe damage to teats on heifers. Mastitis or teat infection in dairy cows also has been associated with horn fly feeding, wherein flies mechanically transmit Staphylococcus aureus and other bacteria that cause mastitis in dairy cattle. Management of horn fly populations can reduce the number of cows suffering from mastitis. Horn flies also are suspected of mechanical transmission of anaplasmosis, anthrax, and other disease-causing pathogens within herds.
The economic thresholds for horn fly presence on cattle are 100 or more per lactating dairy cow or 200 or more per beef cow. In some cases, horn flies are present at extremely high densities. For example, up to 20,000 flies per animal has been reported. In areas where flies are present at such high densities per cow, the blood loss due to feeding also has an impact on production, with the population consuming over a liter of blood per week. In general, horn flies are present at lower population densities on dairy cattle than on beef cattle. The reason for this is that dairy cattle are often fed grain-based feed that reduces the survival of immature horn flies in the manure, resulting in lower fly production.
Treatment of cattle for horn flies is highly beneficial. Weight gain and lactation are consistently lower on animals that are not treated for horn flies. When fly densities reach or exceed the economic thresholds (100 per dairy cow and 200 per beef cow), the cost of treatment is always exceeded by the benefit in terms of cattle health and production. For example, the control of horn flies on beef cattle resulted in increased growth of their calves believed to be due to increased milk production.
Horn flies may blood feed on other hosts such as horses, dogs, pigs, and humans, but the immature stages can only develop in cattle manure.
Monitoring
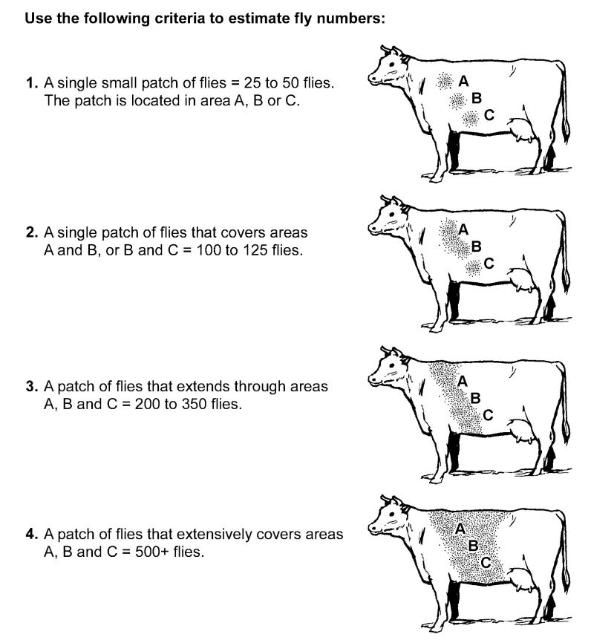
Observing cattle behavior should give a warning of horn fly or other biting fly activity. Cattle being bothered by horn flies will act defensively, licking and twitching their skin, switching their tails, and kicking at their bellies. The number of horn flies per animal may be easily estimated in the field under sunlit or shade conditions. Animals should be selected randomly, and estimates should be made for 10 animals per herd (Figure 6). Experimental results indicate that 50 flies per side on a dairy cow or 100 flies per side on a beef animal is the economic threshold.
The criteria in Figure 6 should be used for estimating fly numbers.
Credit: UF/IFAS, Source: ENY-285/IG137 "Horn Flies"
Control
The close association of horn flies with cattle (the flies leave the host only to lay eggs or to relocate to a new animal) makes horn flies particularly suitable for on-animal chemical control measures. However, in Florida and other states, the flies have developed resistance to many of the insecticides found in ear tag formulations. Check with your local Cooperative Extension Service Educator to determine which treatments should be used for horn fly control in your area.
Several different methods can be used to apply insecticide to cattle for horn fly control, but ear tags and forced-use dust bags provide the best control. Self-treatment methods such as dust bags and back rubbers will provide control, although backrubbers are usually less successful on horn flies (Figure 7). For self-treatment devices to be effective, cattle must be "forced" to use them. For example, dust bags may be hung in exit alleyways from barns or placed between pasture and water or feed sources.
See ENY-279 Self-Treatment Methods for Livestock—Backrubbers and ENY-281 Management of External Parasites with Forced-Use Dust Bags for information on the use of self-treatment options for horn fly control.
Sprays and dips also may be used successfully for horn fly control. Application of residual sprays must reach 1–2 quarts per animal at 150 to 200 psi to ensure complete coverage of the animal and penetration to the skin. Animals should be treated in small groups to ensure that all animals receive an even application of the treatment.
The immature stages of the fly may be controlled in the manure by the application of feed-through additives. However, the effect of immature control may not result in a decline in the adult population because flies emigrate from neighboring areas.
A walk-through trap is a non-chemical method of control that collects and kills horn flies directly from cattle. The animals must be "forced" to pass through the trap regularly as with the other self-treatment options described above.

Credit: P. E. Kaufman, UF/IFAS
Keys to Pesticide Safety
- Before using any pesticide, stop and read the precautions.
- Read the label on each pesticide container before each use. Heed all warnings and precautions.
- Store all pesticides in their original containers away from food or feed.
- Keep pesticides out of the reach of children, pets, and livestock.
- Apply pesticides only as directed.
- Dispose of empty containers promptly and safely.
Recommendations in this document are guidelines only. The user must insure that the pesticide is applied in strict compliance with label directions.
The Food and Drug Administration has established residue tolerances for certain insecticides in the meat of certain animals. When these and other approved insecticides are applied according to recommendations, the pests should be effectively controlled and the animals' products will be safe for consumption.
The improper use of insecticides may result in residue in milk or meat. Such products must not be delivered to processing plants. To avoid excessive residues, use the insecticides recommended at the time recommended and in the amounts recommended.
See ENY-272 Pesticide Safety around Animals for information on how to correctly and safely treat livestock with insecticides.
Locating an Approved Pesticide
In 2014, a group of livestock entomologists, as a part of Multistate Hatch Project S-1060, developed an online system for obtaining the names of registered pesticides appropriate for use with livestock and pets. This is a state-specific database (only certain states are represented, and Florida is one of these); if you are in another state, you must be certain that your state is represented in the dropdown list.
This database is easily searchable by the type of animal or site that you want to treat (such as a barn), as well as the targeted pest. From these two selections, you can then choose the "Method of Application" and the "Formulation Type." To use this system, please visit the following website http://veterinaryentomology.ucr.edu/vet_pesticides.html.
Although we continuously strive to keep this database current, it is ultimately your responsibility to ensure that the product that you choose is registered in Florida (and the application is made in Florida) and that you use the product in accordance with the label requirements and local laws and ordinances. Remember, "the label is the law" for pesticide use, and the uses indicated on the label, including the site of application and targeted pest(s) must be on the label.
If you have any challenges with this system, please contact your local UF/IFAS Extension office (http://sfyl.ifas.ufl.edu/map/index.shtml) or for additional assistance contact Dr. Phillip Kaufman, pkaufman@ufl.edu.
See ENY-274 External Parasites on Beef Cattle and ENY-251 External Parasites on Dairy Cattle for specific control information.
Selected References
Byford, R. L., M. E. Craig, and B. L. Crosby. 1992. "A review of ectoparasites and their effect on cattle production." J. Anim. Sci. 70: 597–602. https://doi.org/10.2527/1992.702597x
Cupp, E. W., M. S. Cupp, J. M. C. Ribeiro, and S. E. Kunz. 1998. "Bloodfeeding strategy of Haematobia irritans (Diptera: Muscidae)." J. Med. Entomol. 35: 591–595. https://doi.org/10.1093/jmedent/35.4.591
Foil, L. D., and J. A. Hogsette. 1994. "Biology and control of tabanids, stable flies and horn flies." Rev. Sci. Tech. 13: 1125–1158. https://doi.org/10.20506/rst.13.4.821
Hogsette, J. A., D. L. Prichard, and J. P. Ruff. 1991. "Economic effects of horn fly (Diptera: Muscidae) populations on beef cattle exposed to three pesticide treatment regimes." J. Econ. Entomol. 84: 1270–1274. https://doi.org/10.1093/jee/84.4.1270
Krafsur, E. S., and C. M. Ernst. 1986. "Phenology of horn fly populations (Diptera: Muscidae) in Iowa, USA." J. Med. Entomol. 23: 188–195. https://doi.org/10.1093/jmedent/23.2.188
Mendes, J., and A. X. Linhares. 1999. "Diapause, pupation sites and parasitism of the horn fly, Haematobia irritans, in south-eastern Brazil." Med. Vet. Ent. 13: 180–185. https://doi.org/10.1046/j.1365-2915.1999.00155.x
Moon, R. D. 2002. Muscoid flies (Muscidae), pp. 45–65. In G. R. Mullen and L. A. Durden (eds.), Medical and Veterinary Entomology, vol. 2. Elsevier, San Diego, CA.
Owens, W. E., S. P. Oliver, B. E. Gillespie, C. H. Ray, and S. C. Nickerson. 1998. "Role of horn flies (Haematobia irritans) in Staphylococcus aureus-induced mastitis in dairy heifers." Am. J. Vet. Res. 59: 1122–1124. https://doi.org/10.2460/ajvr.1998.59.09.1122
Pruett, J.H., C. D. Steelman, J. A. Miller, J. M. Pound, and J. E. George. 2003. "Distribution of horn flies on individual cows as a percentage of the total horn fly population." Vet. Parasitol. 116: 251–258. https://doi.org/10.1016/j.vetpar.2003.07.004
Watson, D. W., S. M. Stringham, S. S. Denning, S. P. Washburn, M. H. Poore, and A. Meier. 2002. "Managing the horn fly (Diptera: Muscidae) using an electric walk-through fly trap." J. Econ. Entomol. 95: 1113–1118. https://doi.org/10.1093/jee/95.5.1113