Introduction
Radopholus similis, the burrowing nematode, is the most economically important nematode parasite of banana in the world. Infection by burrowing nematode causes toppling disease of banana, yellows disease of pepper, and spreading decline of citrus. These diseases are the result of burrowing nematode infection destroying root tissue, leaving plants with little to no support or ability to take up water and translocate nutrients. Because of the damage that it causes to citrus, ornamentals, and other agricultural industries worldwide, burrowing nematode is one of the most regulated nematode plant pests (Hockland et al. 2006).
Distribution
Burrowing nematode is native to Australasia, but is found worldwide in tropical and subtropical regions of Africa, Asia, Australia, North and South America, and many island regions. The widespread range of this nematode is due to its dissemination with propagative plant material, especially infected banana corms (O'Bannon 1977; Gowen et al. 2005). Unlike most other plant-parasitic nematodes, the influence of soil texture on burrowing nematode population levels varies with the host (Chabrier et al. 2010). Radopholus similis infection on citrus is favored by coarse sandy soil that is poor in organic matter, but is hindered by fine textured soils rich in organic matter. However, soil texture plays a less important role on nematode population levels on banana (O'Bannon 1977).
Life Cycle and Biology
Burrowing nematode is an endoparasitic migratory nematode, meaning it completes its life cycle within root tissue. All motile juvenile stages and females can infect root tissue at any point along the length of a root. After root penetration, these life stages mainly feed and migrate into the cortical parenchyma and also into the stele. Mature males of burrowing nematode are not infective. As the mature females migrate through root tissue, they lay eggs that are produced through either sexual reproduction with males or by hermaphroditistim (Thorne 1961; Kaplan and Opperman 2000). Once an egg hatches, the emergent second-stage juvenile can migrate within the root and complete its entire life cycle within the root system, or it can leave the roots in search of another healthy host root. Progeny of a single individual have been observed congregating within the maternal migration path and causing localized areas of heavy damage that often lead to the death of the infected root (Figure 1, Thorne 1961).
Credit: John H. O'Bannon, Florida Department of Agriculture and Consumer Services (deceased)
Symptoms
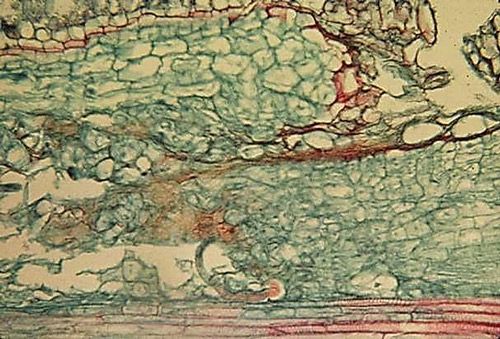
Symptoms of burrowing nematode are most readily observable as dark and necrotic lesions on the root system (Figure 2), similar to those caused by pathogenic fungi, Helicotylenchus multicinctus, and other endoparasitic nematodes that may infect banana roots. Root systems can become stunted, unthrifty, and necrotic (Figure 3). In banana, lesions may be present in both the roots and outer layer of the rhizome. On citrus in Florida, root damage is more severe in deep, coarse, sandy soil.

Credit: Robert Dunn, UF/IFAS Entomology and Nematology Department (retired)

Credit: Robert Dunn, UF/IFAS Entomology and Nematology Department (retired)
Citrus roots that are growing within the nematode-conducive sandy soil have few to no small feeder roots and are severely damaged by the nematode feeding and tunneling. Aboveground symptoms of citrus and other plants infected by burrowing nematode include yellowing, stunting, dieback, reduced fruit size, and thinning of the canopy (Figure 4). Spreading decline of citrus can begin in small areas of citrus groves where a few trees demonstrate reduced thriftiness, canopy thinning near the crown, and a reduction in fruit size. Banana toppling occurs when heavy infestations cause trees to fall over during periods of heavy rain or wind due to their severely reduced and damaged root system, which is unable to anchor the plant into the ground (Figure 5). Pepper fields suffering from burrowing nematode infestation will display yellowing symptoms coupled with root necrosis and canopy dieback (Thorne 1961).

Credit: Nicholas Sekora, UF/IFAS Entomology and Nematology Department

Credit: Danny Coyne, Consultative Group on International Agricultural Research
Hosts
Burrowing nematode has been observed infecting more than 300 plant species including banana, citrus, coffee, coconut, ginger, pepper, sugarcane, tea, and several ornamentals (Thorne 1961; Orton Williams and Siddiqi 1973). Burrowing nematode parasitizes citrus only in Florida. Two host races were proposed based on differential infection and reproduction on banana and citrus of Radopholus similis populations from Florida (DuCharme and Birchfield 1956). These two races were later split into two separate species based on morphological, biochemical, and cytological differences: Radopholus similis that does not infect citrus and Radopholus citrophilus that can infect citrus (Huettel and Yaegashi 1988). Today, these two species are again classified as the "banana race" and "citrus race," respectively, within Radopholus similis. Both races infect banana and other host plants; only the citrus race infects citrus.
Identification
Burrowing nematode exhibits marked sexual dimorphism (Figure 6). Male nematodes possess a raised lip region and a reduced feeding apparatus (stylet and esophagus) because they are not infective (Figure 7A). The tail of male burrowing nematode has a distinctive bursa extending at least two-thirds of the tail length that it uses to clasp the female body during mating (Figure 7B). Females do not have a raised lip region, but do have a heavily sclerotized and thickened framework (Figure 8A). The female stylet is robust with three distinct knobs. The vulva, the opening of the reproductive system, is located slightly below mid body (Figure 8B).
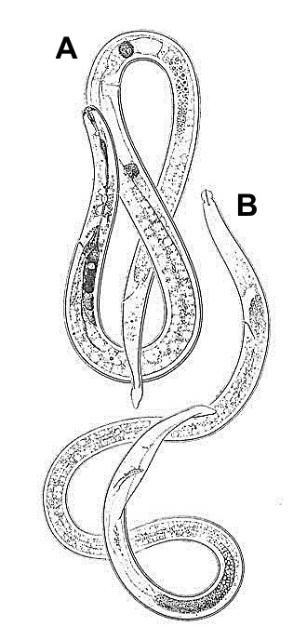
Credit: Figure used with permission from Mohammed R. Siddiqi. CABI Bioscience
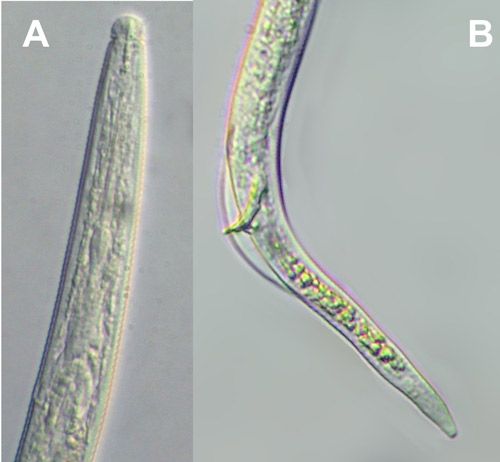
Credit: Nicholas Sekora, UF/IFAS Entomology and Nematology Department
![Figure 8 Figure 8. Radopholus similis female head region (A) and full body with vulva [v] near midbody (B).](/image/IN969/7812176/2345114/2345114-2048.webp)
Credit: Nicholas Sekora, UF/IFAS Entomology and Nematology Department
Despite attempts to separate the two races of burrowing nematode, it is impossible to differentiate between the banana and citrus races without host testing. Morphology during and after development and advanced microscope and molecular methods were used to separate the two races (Huettel and Dickson 1981; Huettel, et al. 1983; Huettel and Yaegashi 1988). However, subsequent research failed to confirm the results of this race differentiation work (Kaplan and Opperman 1997 and 2000).
Economic Importance
Burrowing nematode parasitizes banana in nearly every country of the world with a tropical to subtropical climate except the Canary Islands, Israel, and Taiwan. The lack of new land free from the burrowing nematode in banana-producing countries has prevented the exclusion of the nematode from new banana plantations and has caused the persistence of nematode problems, which result in crop losses ranging 30%–80% (Gowen et al. 2005). In Florida, the citrus race of the burrowing nematode causes spreading decline symptoms only in the deep and coarse sandy soil of the Ridge in central Florida, where yield losses range 40%–80% (Duncan 2005). The spreading decline symptoms are not observed in Florida citrus areas that are established in fine textured soils or in the organic flat woods soils of central-southern Florida, even in the presence of the nematode.
Management
Many states within the United States and countries around the world have heavy import-export regulations on the movement of burrowing nematode. These phytosanitary regulations were enacted mainly by citrus and ornamental producing states and countries in order to protect their citrus and ornamental industries from the introduction of the citrus race of the nematode. The state of Florida, where the citrus race occurs, implements internal regulations and a citrus nursery certification program in order to prevent the dissemination of burrowing nematode with infected propagative citrus material. Additional ornamental certification programs are enacted to enable the Florida ornamental industry to export plants into national and international markets that regulate burrowing nematode.
Preplant management practices after the removal of infected banana or citrus trees can have a significant impact on reducing and delaying reinfection of replanted trees. Mechanical residue removal is widely used, but can spread infected material. By chemically killing a plant before removing it from a field, it may be possible to drastically reduce population levels. Additional nematicidal treatments to the soil and weed control in fallow fields can further reduce populations of burrowing nematode during the typical one-year fallow period used for banana cultivation (Chabrier and Quénéhervé 2003). Alternatively, studies utilizing fungal endophytes that live within plants have shown some promise by inducing resistance to burrowing nematode in banana (Vu et al. 2006).
While nematicides available for management of burrowing nematode have been continuously reduced, the only consistent means of management beyond cultural practices is the use of clean and resistant root stock or propagative material. Nematode-free banana corms are obtained from tissue culture, but unless planted in land free from burrowing nematode this only delays infection in nematode-infested soils. The citrus nursery certification program implemented in Florida allows the production of clean seedlings, which when planted in land free of burrowing nematode, prevents nematode problems. Resistant and clean rootstocks (Carrizo Citrange) have been used successfully in replanting citrus orchards in the Florida Ridge, but resistance-breaking nematode populations have been detected (Kaplan and O'Bannon 1985; Kaplan 1986).
Selected References
Chabrier C, Quénéhervé P. 2003. "Control of the burrowing nematode (Radopholus similis Cobb) on banana: Impact of the banana field destruction method on the efficiency of the following fallow." Crop Protection 22: 121–127.
Chabrier C, Tixier P, Duyck P-F, Carles C, Quénéhervé P. 2010. "Factors influencing the survivorship of the burrowing nematode, Radopholus similis (Cobb.) Thorne in two types of soil from banana plantations in Martinique." Applied Soil Ecology 44: 116–123.
Duncan LW. 2005. Nematode parasites of citrus. pp. 437-466. In: Plant Parasitic Nematodes in Subtropical and Tropical Agriculture (Luc M, Sikora RA, Bridge J, eds). CAB International, Wallingford, UK.
Gowen SR, Quénéhervé P, Fogain R. 2005. Nematode parasites of bananas and plantains. pp. 611–643. In: Plant Parasitic Nematodes in Subtropical and Tropical Agriculture (Luc M, Sikora RA, Bridge J, eds). CAB International, Wallingford, UK.
Hockland S, Inserra RN, Millar L, Lehman PS. 2006. International plant health- Putting legislation into practice. pp. 327–345. In: Plant Nematology (Perry RN, Moens M. eds.) CAB International, Wallingford, UK.
Huettel RN, Dickson DW. 1981. "Parthenogenesis in the two races of Radopholus similis from Florida." Journal of Nematology 13: 13–15.
Huettel RN, Dickson DW, Kaplan DT. 1983. "Biochemical identification of the two races of Radopholus similis by starch gel electrophoresis." Journal of Nematology 15: 338–344.
Huettel RN, Yaegashi T. 1988. "Morphological differences between Radopholus citrophilus and R. similis." Journal of Nematology 20: 150–157.
Kaplan DT, O'Bannon JH. 1985. "Occurrence of biotypes in Radopholus citrophilus." Journal of Nematology 17: 158–162.
Kaplan DT. 1986. "Variation in Radopholus citrophilus population densities in the citrus rootstock Carrizo Citrange." Journal of Nematology 18: 31–34.
Kaplan DT, Opperman CH. 1997. "Genome similarity implies that citrus-parasitic burroeing nematodes do not represent a unique species." Journal of Nematology 29: 430–440.
Kaplan DT, Opperman CH. 2000. "Reproductive strategies and karyotype of the burrowing nematode, Radopholus similis." Journal of Nematology 32: 126–133.
McSorley R. 1986. Nematode problems on bananas and plantains in Florida. Nematology Circular No. 133, pp 4. Florida Department of Agriculture and Consumer Services, DPI.
O'Bannon JH. 1977. "Worldwide dissemination of Radophilus similis and its importance in crop production." Journal of Nematology 9: 16–25.
Orton Williams KJ, Siddiqi MR. 1973. Radopholus similis C.I.H. Description of Plant-Parasitic Nematodes. Set 2, No. 27, pp. 4. Commonnwealth Institute of Helminthology, St. Albans, Herts, UK.
Thorne G. 1961. Principles of Nematology. New York, NY: McGraw-Hill Book Company, Inc.
Vu T, Hauschild R, Sikora RA. 2006. "Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana." Nematology 8: 847–852.