Introduction
Rice blast disease is one of the most devastating fungal diseases that can impact more than 50 species of grasses, including rice, barley, wheat, oat, and millet (Zhang et al. 2022). Found in over 85 countries, rice blast poses a serious threat to global food security, annually destroying approximately 10% to 30% of harvested rice (Nalley et al. 2016). This amount would otherwise be enough to feed 60 million people (Pennisi 2010). As the global human population is projected to surpass 9.7 billion by 2050, affordable high-calorie foods such as rice will become pivotal to keep up with food demands. However, with a projected 30% increase in global rice production required by 2035 to meet these growing demands, it is important to implement effective agricultural practices for sustaining and enhancing rice production (Prasad et al. 2017). The purpose of this document is to increase awareness of one of the most highly destructive plant pathogens by describing its lifestyle, symptoms, and current disease mitigation practices. Given that the Everglades Agricultural Area (EAA) plays a vital role in rice cultivation, this publication is also intended for Florida rice growers to be used as a diagnostic field guide in the identification and management of rice blast disease.
Pathogen
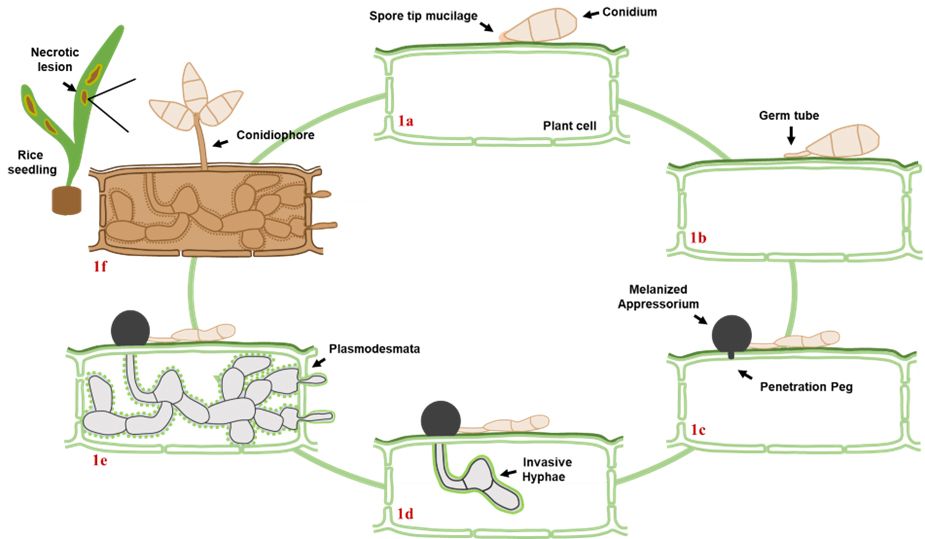
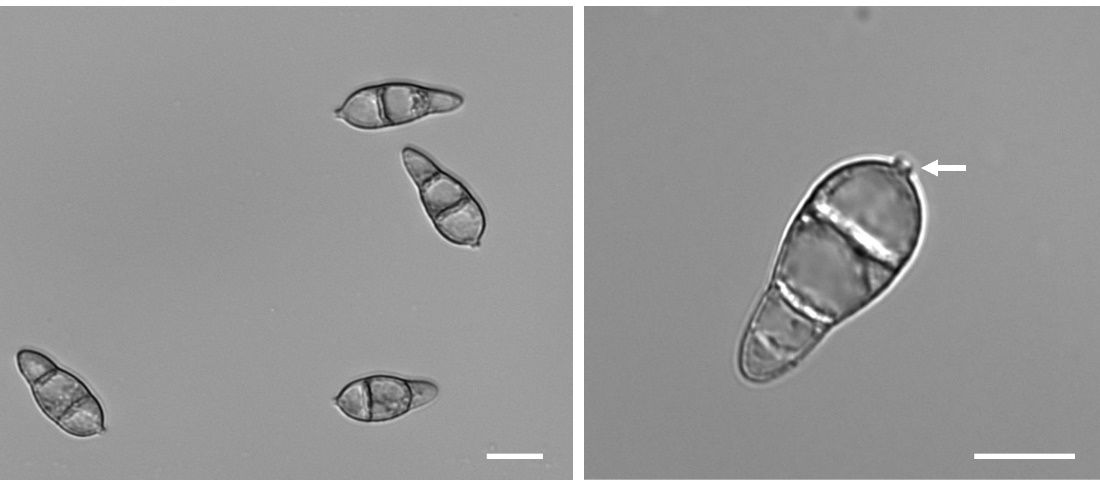
Magnaporthe oryzae (synonym Pyricularia oryzae) is the causal agent responsible for rice blast disease. M. oryzae is a branching, filamentous fungus that belongs to the Ascomycota phylum and the Pyriculariaceae family. This fungus is a hemibiotrophic fungus, meaning it has two distinct phases of living during the infection process (Figure 1). Initially, M. oryzae enters a more friendly, biotrophic phase, where the fungus invades and lives inside the host cells without causing immediate damage. During this phase, it avoids triggering the plant’s immune response, allowing the fungus to spread undetected. After a few days, M. oryzae switches to a harmful necrotrophic phase, where it starts killing and feeding on host cells, leading to the formation of visible necrotic lesions on the surface of the leaf (Fernandez and Orth 2018). These necrotic lesions are the sites of cell death on the leaf, appearing as brown, darkened spots. On these necrotic lesions, M. oryzae produces thousands of asexual, self-replicating spores known as conidia that are instrumental in continuing the fungal life cycle. M. oryzae conidia are three-celled and have an elongated shape (Figure 2). They are often seen as a dark-brown color and are approximately 20–30 micrometers long — this is roughly half the thickness of a human hair. M. oryzae conidia can produce a sticky substance, called mucilage, that helps with the adherence to the surface of the leaf (Hamer et al. 1988).
Credit: J. Fernandez, UF/IFAS
Credit: R. Kalicharan, UF/IFAS
Disease Cycle
Rice blast disease begins when the conidium lands on the leaf’s surface, attaches itself to the leaf cuticle, and germinates (Figure 1) (Cruz-Mireles et al. 2021). Day temperatures of 25°C–28°C (77°F–82°F), night temperatures of 17°C–23°C (63°F–73°F), and humidity exceeding 90% are optimal for spore germination. Proper germination leads to the production of a penetration structure known as an appressorium. The appressorium is a dome-shaped structure that generates massive amounts of turgor pressure, caused by an accumulation of the compound glycerol, to allow for mechanical penetration of the leaf cuticle (outermost leaf surface). The appressorium is surrounded by a thick layer of dark pigmentation known as melanin that prevents the movement of water outside the cell and helps build enough turgor pressure (Wang et al. 2005). M. oryzae utilizes a structure known as a penetration peg generated at the bottom of the appressorium to direct the mechanical force through the plant cuticle (Wilson and Talbot 2009). Once inside the initial plant cell, M. oryzae produces long branching filaments known as invasive hyphae (fungal cells) that allow the fungus to disperse within a plant cell and move to neighboring cells via plant connections known as plasmodesmata (Kankanala et al. 2007). During this initial biotrophic stage of infection, the fungus is able to colonize plant cells in a way that the natural plant cell defense response is not activated (Fernandez 2023). This makes it difficult to identify the initial stages of the rice blast disease in the field. After 4–5 days, the fungus switches to its necrotrophic phase, where it begins killing the cells it has colonized and produces visible necrotic lesions on the leaf surface (Perfect and Green 2001).
Symptoms
M. oryzae can infect plants of all ages, from seedlings to mature plants. Rice blast can also infect various parts of a plant including leaves, sheath, neck, and panicle (Puri et al. 2009). Most notably, rice blast disease presents distinct physical symptoms, including lesions that appear on the surface of the leaf. These lesions initially begin as small spots and progress into diamond-shaped formations, featuring a dark-brown color with lighter-colored, yellowish leaf tissue around the margins (Figure 3). Rice cultivars more resistant to rice blast will produce smaller and darker lesion types compared to more susceptible cultivars. While less common, symptoms on the sheath also produce the same type of necrotic lesions.
One of the most severe symptoms of rice blast is infection to the neck. When M. oryzae sporulates at the stem of the plant, the stem becomes weak and likely to break; this is known as neck rot. Neck rot symptoms can prevent nutrient and water flow to the panicle, a specialized plant structure that eventually produces rice grains (Bonman 1989). This may prevent adequate maturation of the grains, which begins to turn the panicle white, or may prevent the development of the panicle entirely. Rice blast can also infect the panicles, producing symptoms such as necrotic lesions. Infected panicles can turn white and be unable to produce viable seeds, further contributing to overall yield loss (Hayashi et al. 2019).

Credit: N. Garcia, UF/IFAS
Dissemination and Spread
The necrotic lesions become sites where the fungus will produce a specialized filamentous cell known as a conidiophore that will produce thousands of spores that can remain viable for up to a week on the leaf surface (Figure 1). These spores can be dispersed through wind and rain to uninfected neighboring plants, allowing the disease cycle to continue (Zhang et al. 2014). Continued disease cycles create a buildup of disease-causing inoculum (the material, such as fungal spores, that can cause infection) in the crop area, making the management of rice blast difficult. In addition, M. oryzae can survive between growing seasons within infected crop residues in the field and/or in contaminated seeds that can introduce the disease to new areas (Raveloson et al. 2018). Environmental conditions such as high humidity, wet weather, and excessive fertilization contribute to the dissemination of M. oryzae spores (Younas et al. 2023). In Florida, typical environmental conditions that prevail during the rice growing season are characterized by high humidity and wet conditions, which promote the germination and dissemination of M. oryzae spores. Unfortunately, regional geographical and climatic conditions amplify the risk of rice blast disease outbreaks.
Cultural Practices
Providing around 20% of the calories consumed worldwide, rice is the most common staple food in the world. In Florida, the Everglades Agricultural Area (EAA) commercially grows thousands of acres of cultivated rice every year (Bhadha et al. 2023). The successful growth of rice crops is tied to several factors including adequate soil structure, crop rotations, and the practice of flooding fields. The soils of the EAA contain about 85% organic matter and are rich in nutrients, providing crops with a suitable environment for efficient nutrient absorption. Crop rotations between rice and sugarcane allow for the retention of soil nutrients throughout growing seasons. In addition, the practice of flooding fields, where large amounts of water are added to inundate entire fields, also reduces nutrient depletion and aids in controlling pests and weeds. In the EAA, flooding is done continuously, from 14–21 days before harvest. Water levels in the field always remain 3–4 inches deep (Bhadha et al. 2018). While many practices are implemented in the EAA to prevent disease progression, Florida rice growers face the persistent threat of rice blast disease negatively impacting rice crop production.
Disease Management
Given that rice blast disease outbreaks have been found in over 80 countries where rice is cultivated, crop rotation is an effective strategy to break the disease cycle, as planting non-host crops in between rice cultivation seasons disrupts the pathogen's continuity (Kato 2001). While not an essential nutrient for rice crops, silicon treatment has been known to reduce rice blast disease in rice by strengthening the plant cell wall, and to aid in plant growth by helping curb abiotic, environmental stresses such as metal toxicity (Datnoff et al. 1997). In Florida, silicon supplementation in areas where silicon content is low has been applied for decades to increase rice yields.
In addition, fungicides are also a key component of chemical control strategies against rice blast disease. Timely application of fungicides, particularly during periods of high disease pressure, helps prevent and manage outbreaks. Synthetic fungicides such as carpropamid, tricyclazole, pyroquilon, and phthalide have been found to be effective against rice blast disease (Arif Khan et al. 2022). However, prolonged use of one fungicide leads to resistance by M. oryzae.
Beyond chemical control strategies, selecting resistant rice varieties is crucial for disease management because it is the most effective way to mitigate rice blast (Miah et al. 2013). Breeding programs that develop rice varieties with inherent resistance to specific races (genetic variants) of M. oryzae contribute significantly to sustainable disease control (Tian et al. 2022). However, through time, the effectiveness of resistant cultivars diminishes due to the evolution of M. oryzae (Syauqi et al. 2022). There is a continual effort to identify and deploy new rice varieties with durable resistance to multiple races of rice blast that can be adapted to local conditions in Florida. Every year, the EAA performs rice variety trials that have allowed for the implementation of rice varieties from different parts of the United States. As such, rice variety diversity has increased from three varieties to eleven from 2008–2021. While this publication does not extensively cover rice production and varieties in Florida, more information can be found in Ask IFAS publication SL439 (https://edis.ifas.ufl.edu/publication/SS653). By incorporating more durable resistant cultivars, farmers can reduce the reliance on chemical inputs and enhance overall field resilience. However, it is essential to regularly assess and update resistance breeding programs to stay ahead of evolving M. oryzae strains.
Diagnosis
The accurate diagnosis of rice blast disease depends heavily on identifying the specific fungal pathogen involved. Initial identification is typically done by direct examination of fungal spores observed on plant tissues under a microscope. Alternatively, inducing sporulation by incubating infected tissue in a moist chamber can aid in identification. Moreover, the pathogen can be isolated and cultured on artificial media, facilitating further examination through the formation and characterization of spores.
As an additional resource, the Florida Extension Plant Diagnostic Clinic (FEPDC) network is available for assistance with pathogen identification. To learn more about sample submission procedures and laboratory diagnosis costs, reach out to your local UF/IFAS Extension office or contact FEPDC directly.
Summary
Rice blast disease, a serious threat to global agriculture, is caused by the filamentous fungus Magnaporthe oryzae. Infection begins when a three-celled, tear-shaped spore lands on the surface of a rice leaf and germinates. For 4–5 days, M. oryzae infects rice cells without causing visible symptoms (the biotrophic phase). Once the rice blast fungus switches to the necrotrophic growth phase, cell death begins, and lesions are visible on the leaf surface. Spores produced on these lesions can be spread via wind or rain and can infect surrounding plants. As rice blast can infect all parts of a plant, it is crucial that measures be taken to try to prevent the spread of M. oryzae, namely through resistant cultivars. Currently, a cultivar that can resist all genetic variants (strains) of M. oryzae does not exist. However, to ensure the sustainability of Florida rice cultivation, an integrated and adaptive approach that combines cultural practices, chemical control, deployment of resistant cultivars, and vigilant monitoring is essential. By implementing these strategies, we can better mitigate rice blast while promoting sustainable and resilient rice cultivation in Florida.
References
Arif Khan, M., M. A. Al Mamun Khan, A. M. U. B. Mahfuz, J. M. Sanjana, A. Ahsan, D. R. Gupta, M. N. Hoque, and T. Islam. 2022. “Highly Potent Natural Fungicides Identified in Silico against the Cereal Killer Fungus Magnaporthe oryzae.” Scientific Reports 12:20232. https://doi.org/10.1038/s41598-022-22217-w
Bhadha, J. H., M. T. VanWeelden, J. M. McCray, R. Cherry, J. Beuzelin, R. N. Raid, and S. Mukhopadhyay. 2018. Rice Production in Florida: A Handbook. 1–42. University of Florida Institute of Food and Agricultural Sciences. https://erec.ifas.ufl.edu/media/erecifasufledu/docs/Rice-Handbook_2018.pdf
Bhada, J. H., L. Trotta, D. Cavazos, and M. VanWeelden. 2023. "Trends in Florida Rice Production and Varieties." SL439. EDIS. https://edis.ifas.ufl.edu/publication/SS653
Bonman, J. 1989. "Leaf and Neck Blast Resistance in Tropical Lowland Rice Cultivars." Plant Dis. 73:388–390. https://doi.org/10.1094/PD-73-0388
Cruz-Mireles, N., I. Eisermann, M. Garduño-Rosales, C. Molinari, L. S. Ryder, B. Tang, X. Yan, and N. J. Talbot. 2021. "The Biology of Invasive Growth by the Rice Blast Fungus Magnaporthe oryzae." Magnaporthe oryzae: Methods and Protocols. 19–40. https://doi.org/10.1007/978-1-0716-1613-0_2
Datnoff, L., C. Deren, and G. Snyder. 1997. "Silicon Fertilization for Disease Management of Rice in Florida." Crop Protection 16:525–531. https://doi.org/10.1016/S0261-2194(97)00033-1
Fernandez, J. 2023. "The Phantom Menace: Latest Findings on Effector Biology in the Rice Blast Fungus." aBIOTECH 4:140–154. https://doi.org/10.1007/s42994-023-00099-4
Fernandez, J., and K. Orth. 2018. "Rise of a Cereal Killer: The Biology of Magnaporthe oryzae Biotrophic Growth." Trends in Microbiology 26:582–597. https://doi.org/10.1016/j.tim.2017.12.007
Hamer, J. E., R. J. Howard, F. G. Chumley, and B. Valent. 1988. "A Mechanism for Surface Attachment in Spores of a Plant Pathogenic Fungus." Science 239:288–290. https://doi.org/10.1126/science.239.4837.288
Hayashi, K., T. Yoshida, and Y. Hayano-Saito. 2019. "Detection of White Head Symptoms of Panicle Blast Caused by Pyricularia oryzae Using Cut-Flower Dye." Plant Methods 15:1–9. https://doi.org/10.1186/s13007-019-0548-z
Kankanala, P., K. Czymmek, and B. Valent. 2007. "Roles for Rice Membrane Dynamics and Plasmodesmata during Biotrophic Invasion by the Blast Fungus." The Plant Cell 19:706–724. https://doi.org/10.1105/tpc.106.046300
Kato, H. 2001. "Rice Blast Disease." Pesticide Outlook 12:23–25.
Miah, G., M. Y. Rafii, M. R. Ismail, A. B. Puteh, H. A. Rahim, R. Asfaliza, and M. A. Latif. 2013. "Blast Resistance in Rice: A Review of Conventional Breeding to Molecular Approaches." Molecular Biology Reports 40:2369–2388. https://doi.org/10.1007/s11033-012-2318-0
Nalley, L., F. Tsiboe, A. Durand-Morat, A. Shew, and G. Thoma. 2016. "Economic and Environmental Impact of Rice Blast Pathogen (Magnaporthe oryzae) Alleviation in the United States." PloS one 11:e0167295. https://doi.org/10.1371/journal.pone.0167295
Pennisi, E. 2010. "Armed and Dangerous." Science (New York, NY) 327:804–805. https://doi.org/10.1126/science.327.5967.804
Perfect, S. E., and J. R. Green. 2001. "Infection Structures of Biotrophic and Hemibiotrophic Fungal Plant Pathogens." Molecular Plant Pathology 2:101–108. https://doi.org/10.1046/j.1364-3703.2001.00055.x
Prasad, R., Y. S. Shivay, and D. Kumar. 2017. “Current Status, Challenges, and Opportunities in Rice Production.” In Rice Production Worldwide, edited by B. Chauhan, K. Jabran, and G. Mahajan. Springer, Cham. https://doi.org/10.1007/978-3-319-47516-5_1
Puri, K. D., S. M. Shrestha, G. B. Khhatri Chhetri, and K. D. Joshi. 2009. "Leaf and Neck Blast Resistance Reaction in Tropical Rice Lines under Green House Condition." Euphytica 165:523–532. https://doi.org/10.1007/s10681-008-9771-9
Raveloson, H., I. Ratsimiala Ramonta, D. Tharreau, and M. Sester. 2018. "Long‐Term Survival of Blast Pathogen in Infected Rice Residues as Major Source of Primary Inoculum in High Altitude Upland Ecology." Plant Pathology 67:610–618. https://doi.org/10.1111/ppa.12790
Syauqi, J., R.-K. Chen, A.-H. Cheng, Y.-F. Wu, C.-L. Chung, C.-C. Lin, H.-P. Chou, H.-Y. Wu, J.-Y. Jian, C.-T. Liao, C.-C. Kuo, S.-C. Chu, Y.-C. Tsai, D.-J. Liao, Y.-P. Wu, A. L. Abadi, L. Sulistyowati, and W.-C. Shen. 2022. "Surveillance of Rice Blast Resistance Effectiveness and Emerging Virulent Isolates in Taiwan." Plant Disease 106:3187–3197. https://doi.org/10.1094/PDIS-12-21-2806-RE
Tian, D., Y. Deng, X. Yang, G. Li, Q. Li, H. Zhou, Z. Chen, X. Guo, Y. Su, Y. Luo, and L. Yang. 2022. "Association Analysis of Rice Resistance Genes and Blast Fungal Avirulence Genes for Effective Breeding Resistance Cultivars." Frontiers in Microbiology 13. https://doi.org/10.3389/fmicb.2022.1007492
Wang, Z.-Y., J. Jenkinson, L. Holcombe, D. Soanes, C. Veneault-Fourrey, G. Bhambra, and N. Talbot. 2005. "The Molecular Biology of Appressorium Turgor Generation by the Rice Blast Fungus Magnaporthe grisea." Biochemical Society Transactions 33:384–388. https://doi.org/10.1042/BST0330384
Wilson, R. A., and N. J. Talbot. 2009. "Under Pressure: Investigating the Biology of Plant Infection by Magnaporthe oryzae." Nature Reviews Microbiology 7:185–195. https://doi.org/10.1038/nrmicro2032
Younas, M. U., G. Wang, H. Du, Y. Zhang, I. Ahmad, N. Rajput, M. Li, Z. Feng, K. Hu, and N. U. Khan. 2023. "Approaches to Reduce Rice Blast Disease Using Knowledge from Host Resistance and Pathogen Pathogenicity." International Journal of Molecular Sciences 24:4985. https://doi.org/10.3390/ijms24054985
Zhang, H., Z. Wu, C. Wang, Y. Li, and J.-R. Xu. 2014. "Germination and Infectivity of Microconidia in the Rice Blast Fungus Magnaporthe oryzae." Nature Communications 5:4518. https://doi.org/10.1038/ncomms5518
Zhang, H.-f., T. Islam, and W.-d. Liu. 2022. “Integrated Pest Management Programme for Cereal Blast Fungus Magnaporthe oryzae.” Journal of Integrative Agriculture 21:3420–3433. https://doi.org/10.1016/j.jia.2022.08.056