Fertilizer management is an important agronomic practice in sugarcane production. When properly calibrated and timed, fertilizer applications provide essential nutrients for achieving high yields. Sugarcane requires 16 essential nutrients to complete its life cycle successfully. In addition, silicon, although not considered an essential nutrient, can provide additional benefits to the crop.
Sugarcane growers can use several best management practices (BMPs) as tools to help them deliver appropriate quantities of fertilizer nutrients to the crop for maximum nutrient utilization while also achieving economically favorable yields. These BMPs include the adoption of a consistent soil-testing program and the use of a leaf tissue (foliar) analysis program. The use of leaf analysis to assess nutrient needs of sugarcane should not be considered a replacement for a soil-testing program. Leaf analysis provides a snapshot of crop nutritional status at the time of sampling while soil testing provides predictive information about the soil's ability to continue to supply nutrients. Foliar analysis can be used to adjust the next fertilizer or amendment application so that nutrient limitations can be corrected (McCray and Mylavarapu 2020; https://edis.ifas.ufl.edu/ag345). Overall understanding of the crop's nutritional status and fertilization needs will be improved by using both a calibrated soil test and a foliar analysis program. In addition, consideration of other factors (e.g., soil type, previous cropping/tillage history, field drainage properties) that affect plant growth and yield will also enhance one's ability to interpret crop nutrient requirements. This document will focus on sugarcane leaf tissue sampling for the purpose of foliar nutrient analysis and interpretation.
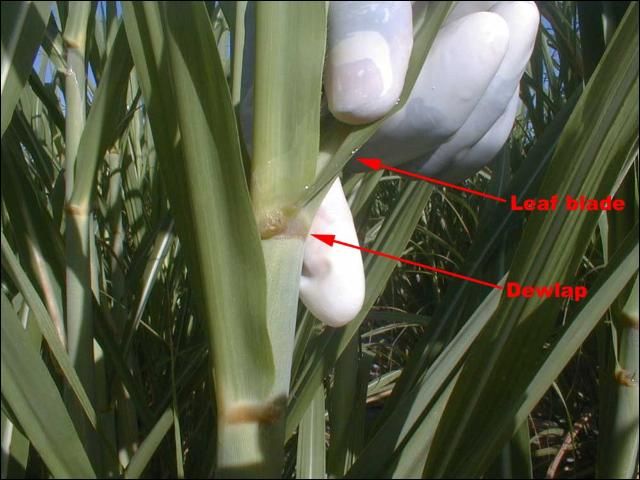
The Top Visible Dewlap (TVD) leaf blade is the plant tissue often used for sugarcane foliar analysis. The TVD leaf is the uppermost fully expanded leaf that has a visible dewlap or distinct collar, a band of membranous tissue between the leaf sheath and the leaf blade (Figure 1). The TVD leaf has been the target leaf that has been sampled to establish foliar analysis standards for sugarcane in Florida. The amount of nutrients in the TVD leaf compared to established standards or critical values will reveal whether the levels of the nutrients are adequate or inadequate for optimal growth and yield. Two methods are used to determine whether the levels of nutrients in the leaves are adequate for growth and yield. These are (1) the Critical Value approach and (2) the Diagnosis and Recommendation Integrated System (DRIS).
In the Critical Value approach the critical concentration of a nutrient is the point at which plant growth is reduced by 5 or 10% from optimum and below which deficiency symptoms appear (Ulrich and Hills 1973). This approach may also include the use of the optimum range, defined as the range of nutrient concentration that is considered optimum for production. Within this range, a particular nutrient is not considered to limit production.
The DRIS calculates nutrient indices relative to zero by comparing leaf nutrient ratios with those of a high-yielding population. This approach incorporates a measure of the relationship between nutrients. It also has the advantage of not being as sensitive to the stage of growth as the Critical Value approach. Thus, DRIS allows a wider time frame during which to collect samples for foliar analysis. (See McCray et al. 2019; https://edis.ifas.ufl.edu/sc075 for more information on using DRIS in Florida sugarcane).
The values or indices obtained from either the Critical Value approach or DRIS depend on whether the midrib is removed from the sampled leaf blade before analysis. The analytical values for most nutrients are lower when the midrib is included in the sample (Muchovej et al. 2005). It is advisable to remove the midrib in the sampling process because most critical values or standard indices have been developed using sampling methods that remove the midrib.
Sampling Procedures
Representative leaf samples must be properly collected to obtain reliable analyses and interpretations. To ensure that good samples are taken and properly handled, particular attention should be paid to organization and preparation before heading to the field to sample leaves. Assemble all the materials necessary for the sampling event (Figure 2), and establish a sampling label protocol that will be easily tracked in the field and laboratory. The following materials are recommended for sugarcane leaf tissue sampling: pre-labeled grocery-sized paper bags to collect whole leaves in the field, distilled water to rinse the leaf blades, and smaller pre-labeled paper bags to store leaf blade samples in the drying oven.

Typically, leaf sampling takes place during the grand growth period of sugarcane (between June and September in south Florida). Leaf samples should be taken from primary shoots or stalks, not from tillers or suckers, and the leaves should be sampled from plants that are representative of the area being sampled. To collect a leaf sample, locate the TVD leaf, hold it near the base, and break the leaf from the sheath with a sharp downward motion. Care should be taken to avoid diseased plants or leaves with insect or herbicide damage since these conditions may affect nutrient levels in the leaves, and the interpretation of results will be compromised.
Routinely, the sampling unit (geographic area to be sampled) should represent the field area that will be managed as a unit that receives similar production and management inputs, generally referred to as a harvest or management unit. Where sugarcane growth is uniform across contiguous blocks (areas between field ditches) and will be treated as one management unit, two or more blocks may be sampled as one unit. Localized areas within a field showing poor growth or other unusual symptoms may need to be sampled separately. Several different sampling units may be identified within a single field, based on localized differences in drainage, soil type, plant health, and/or plant growth. Samples should be taken from plants that are at least 30 ft away from the edge of the field or ditch and at least 100 ft away from the end of the field unless these boundary areas are specific areas of concern. Soil parameters (such as pH) within these field perimeter areas are often modified by in-field ditch and roadway spoils that contain materials that are very different from the overall field soil.
A suggested sampling pattern is to collect 16 leaves in a V-pattern in each of two locations on one end of a field. The V-pattern is accomplished by starting on one side of the block being sampled and moving across one or more rows as each leaf is sampled so that you move diagonally into and out of the field. If you begin sampling 100 ft into a typical commercial-sized sugarcane field and take a sample every 50 ft, this would take you approximately 450 ft into the field to get the first 8 leaves before turning around and collecting 8 leaves on the way back. The two sub-samples of 16 leaves would be combined for a 32-leaf sample for analysis. Depending on uniformity of a field, a grower may decide to collect a separate sample from a particular portion of interest in the field. Sampling the entire length of a quarter or half-mile field should not be necessary to obtain a representative sample of the area. From a management standpoint, it may not be possible to subdivide a management unit, except when it is in line with grower objectives, and fertilizer recommendations can be applied directly to each sub-sampling unit. A grower needs to make the decision on the sampling unit based on the size of the management unit and the specific dimensions of the planted area that contains the plants that exhibit the feature(s) of interest.
During the entire sampling process, it is important that leaves be kept as clean as possible and that they are never placed directly on the ground. After a leaf sample is taken, it should be processed on the same day that it is collected. If processing cannot be done immediately, the sample should be placed in a paper bag, labeled, and kept out of the sun to prevent excessive moisture loss. A better method of keeping samples fresh is to place them in a plastic bag stored inside a large cooler with ice. After removing midribs from leaf blades (Figure 3), leaf blades should be rinsed in distilled water to remove soil and dust particles that may contaminate the samples (Figure 4). Place rinsed samples in dry labeled sample bags ready for drying in the oven (Figure 5). Typically, leaf samples are dried in the oven at 140°F. Depending on how the laboratory receives leaf tissue samples, the tissue may be ground (usually to pass a 1 mm screen) or sent out as dried, un-ground samples.



Summary
TVD leaf sampling and subsequent interpretation of the leaf tissue nutrient analysis are tools that support decisions regarding fertilizer recommendations for optimum growth and yield of sugarcane. Care should be taken to obtain representative leaf samples. Leaf samples should be properly processed to ensure accurate results and interpretation of fertilizer requirements.
References and Further Reading
Anderson, D. L., and J. E. Bowen. 1990. Sugarcane Nutrition. Atlanta, GA: Potash and Phosphate Institute.
Elwali, A. M. O., and G. J. Gascho. 1983. "Sugarcane response to P, K, and DRIS corrective treatments on Florida histosols." Agron. J. 75: 79–83.
Elwali, A. M. O., and G. J. Gascho. 1984. "Soil testing, foliar analysis, and DRIS as guides for sugarcane fertilization." Agron. J. 76: 466–470.
McCray, J. M., I. V. Ezenwa, R. W. Rice, and T. A. Lang. 2019. Sugarcane plant nutrient diagnosis. Gainesville: University of Florida Institute of Food and Agricultural Sciences. SS-AGR-128. https://edis.ifas.ufl.edu/sc075
McCray, J. M., and R. Mylavarapu. 2020. Sugarcane nutrient management using leaf analysis. Gainesville: University of Florida Institute of Food and Agricultural Sciences. SS-AGR-335. https://edis.ifas.ufl.edu/ag345
Muchovej, R. M., P. R. Newman, and Y. Luo. 2005. "Sugarcane leaf nutrient concentrations: with or without midrib tissue." J. Plant Nutrition 28:1271–1286.
Samuels, G. 1969. Foliar Diagnosis for Sugarcane. Chicago: Adams Press. 362 pp.
Ulrich, A., and F. J. Hills. 1973. "Plant analysis as an aid in fertilizing sugar crops: Part 1. Sugar beets." In Soil testing and plant analysis, edited by L. M. Walsh and J. D. Beaton, 271–288. Madison, Wisconsin: SSSA.