The purpose of this publication is to assist with the management of naturally-occurring and fertilizer salts in soils found in the landscape and in farming areas. Management of seawater salts forced onto land by hurricanes is also discussed.
Locations Likely to Have Excessive Salts in Soil
Salts in Florida soils are commonly found in coastal areas, including landscaping at marina-centered housing developments, and where irrigation well draw-down has resulted in saltwater intrusion into the underlying aquifer.
It is also fairly common in Florida to find situations where over-fertilization of crops or landscape plants has resulted in excessive soluble salts in soil. Nutrients supplied by inorganic fertilizers are usually soluble, directly contributing to the salts in the soil. When correct fertilizer rates are used, the salt contribution from fertilizers is low and does not adversely affect crop growth.
If containerized plants are not provided sufficient drainage, then the salts from irrigation water and fertilizers can also be concentrated due to drying of the media during evapotranspiration. A small amount of excess irrigation water should be provided to allow leaching from the container (usually about 20% of the water added). This leaching will ensure that salts do not accumulate and adversely affect the plants. Alternately, over-irrigation will lead to unnecessary leaching of plant nutrients.
Many cities and towns are now using treated effluent water from sewage treatment plants. This reuse of water on crops or golf course fairways and greens is an excellent way of disposing of the water in an environmentally sound manner. However, with each reuse, the salt concentration of the water is often increased. The electrical conductivity of the water should be measured regularly. If elevated readings are found, usually greater than 1.5 to 2.0 deciSiemens per meter, then the water should be mixed with better quality water before it is used for irrigation. If mixing is not possible, then this water should not be used for irrigation except by experienced water managers.
Salts may also be introduced to the soil and aquifers by catastrophic storm events such as hurricanes. Flooding induced by storm surge will directly introduce seawater onto land previously free of salinity problems. Differential hydrologic heads caused by the storm may also produce unfavorable saltwater intrusion, either into the ground water aquifer, the subsoil, or both. When the storm occurs near the end of an area's rainy season, it may take several months for salt removal via the natural rainfall and leaching process.
Salt effects on plants are often exacerbated during periods when drying conditions persist. During drying, water is removed from the soil by evaporation from the soil surface and by transpiration from plants. Salts are left behind that were previously dissolved in the soil water, concentrating them and increasing the adverse impact on plants.
Treatment of Salt-Affected Areas
Land contaminated by salts can usually be reclaimed. Salts must be removed (leached) from the soil profile by high-quality (low-salt) water either from rainfall or irrigation. Water should be added to the soil so that the salts are leached downward below typical rooting zones in the area. The need for leaching implies, of course, that sufficient natural or artificial drainage is present. In most mineral soils of Florida, removal of salts requires only that little additional salt be introduced onto the soil from the water source and that sufficient drainage be provided. If saline water is allowed to remain for substantial periods in a shallow water table beneath the plant root zone, salts can readily move back into the root zone during periods of water table rise or during drying periods through upward soil-capillary movement.
Some clients might be familiar with damage caused by excessive amounts of sodium in soils, particularly in areas of fine-textured soils, such as the Gulf coastal plain of Texas and Louisiana. Sodium-affected soils have not been a problem in Florida. The soils of Florida commonly contain such low amounts of clay that the effect of dispersed clays, if any, is minimal. Florida's high rainfall, coupled with its sandy soils, prevent appreciable sodium effects from being observed. Therefore, gypsum use for lowering of soil sodium levels is not recommended as a general treatment in Florida.
Testing for Elevated Salts in Soil and Water
Before using a water source for landscape or crop irrigation, the water should be tested for pH and electrical conductivity (EC). Water with EC greater than 2.0 deciSiemens per meter (2.0 millimhos per centimeter) generally should not be used for irrigation.
Salt Water Intrusion
Intrusion of salt water into previously nonsaline aquifers is relatively common, especially in shallow aquifers along Florida's coasts. The use of water from these aquifers is more rapid than the recharge rate allowing the water table to fall. Salt water then flows down into the aquifer increasing the salts in the aquifer. An indication of salt water intrusion is an increase in EC with time. Treatment is beyond the scope of this document, but additional water should not be removed from the aquifer, and an alternate water source should be used. Continued pumping from an aquifer during periods of salt water intrusion will result in poorer quality water for longer periods of time.
Storm Events
If the EC has increased significantly following a storm event, the water source may have become contaminated with saltwater. If saltwater has entered the aquifer from above (due to flooded storm water conditions), then the initial water being pumped may contain high salt loads if the pump is extracting water from the salt-affected portion of the aquifer. This saline water should be discharged to drainage systems not used for irrigation. Even if the initial EC following a storm appears acceptable, the EC should be rechecked weekly or bimonthly for a few months, to assure that saline water initially overlaying or adjacent to the well's zone of influence is not drawn to the well over time.
After a period of pumping, the EC of the water may decrease to acceptable irrigation salt levels. If EC readings remain high and prove to be from saltwater intrusion, further pumping is not advised. Removal of water from such an aquifer may cause even further saltwater intrusion.
Soil
The UF/IFAS Extension Soil Testing Laboratory (ESTL) determines EC using a 2:1 solution:soil extraction. This procedure is more time-efficient, results in enough filtered solution for additional tests, and requires less skill to perform accurately. The saturated extract technique on Florida's sandy soils is much more difficult, especially at county laboratory facilities. Standardization among technicians is the biggest problem. For that reason, ESTL continues to use the 2:1 solution:soil extraction procedure.
Historically, the ESTL reported soluble salt values for Florida soils in terms of parts per million (ppm), using a dry-soil basis. This approach works poorly in much of the world, but it served Florida agriculture well, because of the uniformly sandy nature of Florida's mineral surface soils. However, because of its infrequent use elsewhere, the ppm dry-soil convention isolated Florida's growers and ag-industry personnel from the large and steadily growing body of salinity literature world-wide. For this reason, the ESTL has been reporting only EC values (2:1) since 1989.
Container Media
Container media should not be air dried before determining EC. Additionally, a saturated paste extract should be used. Due to the fibrous nature of most container media samples, use of a 2:1 or other ratio-based extract may not result in sufficient solution to properly complete the test.
Specific interpretations (Table 5) have been developed for container media. Readers are cautioned to use only the interpretations found in Table 5 and not those contained in the other tables which address salt effects in mineral soils.
Assumptions and Mode of Reporting EC Values
The saturation extract has been adopted world-wide as the soil water-content standard for such soluble-salt measurements. In general, the water content of soil following drainage of free water from either over-irrigation or a heavy rain (the so-called field capacity ) is ½ to 1/3 that at saturation. Similarly, the water content at the crop's permanent wilting point (from which the crop will not recover even if the soil is rewetted) is ½ to 1/3 that at field capacity. For a reasonably rapid and reproducible estimation of soluble salts for extremely sandy soils such as are common to Florida, it is generally acceptable to use a higher water content for extraction of soluble salts.
Estimation of a saturated extract EC, termed "Salt Index" in the following tables, from the 2:1 extract can be calculated using the following formula:
EC (salt index) = EC (2:1) x 8.
For example, a 0.2 deciSiemens per meter value from the 2:1 procedure is equivalent to a salt index of 1.6 deciSiemens per meter (0.2 x 8 = 1.6). The calculation is completed on the ESTL report form under the column "Estimated Salt Index."
The salt index will vary as rainfall or crop uptake removes soluble salts from the crop-root zone, or as fertilizer salts or salts from irrigation waters are added to the soil. Salt index also normally varies both across the crop bed (depending upon fertilizer placement and subsequent water-movement patterns) and with depth beneath the bed. Conventional wisdom holds that the crop responds to the weighted-average salinity (EC) within the root zone, though this is likely inaccurate for banded fertilizer placement. If sufficient low-salinity water is maintained in the root zone to meet plant evapotranspiration needs, many plants will grow quite well (barring specific-ion effects) despite the presence of moderately- to highly-saline water within their normal rooting depths.
Modification for Soils Containing Lime or Gypsum
Because of the high water content used for soluble-salts estimation at the ESTL, the client must be aware of possible misinformation if the soil sample also contains lime or gypsum. Approximately 25% of the soil samples received by the ESTL in recent years have had pH values high enough to suggest the presence of lime. Even for soils not recently limed, the continued use of irrigation water from limestone aquifers may result in the accumulation of free carbonates in frequently-wetted portions of the profile. Lime is soluble to the extent of approximately 0.1 deciSiemens per meter (millimhos per centimeter) at pH 8, and is progressively more soluble at lower pH values. Because of lime dissolved at 200% water content (a 2:1 water:soil ratio) which would not be in solution at 26% water content (saturation), a correction factor of 1.0 should be subtracted from the salt index for soil samples known to contain free carbonates in addition to their soluble salts.
Gypsum-affected soils in Florida are quite rare and their testing is not described here.
Salt-index Ranges for Selected Commodities
In Tables 1, 2, 3, and 4, crops are grouped from most-sensitive to least-sensitive within a given crop type. Numbers in the third column, labeled "Salinity effect threshold (dS/m)," indicate the salt index at which salts begin to affect the plant's growth. Values in the fourth column, labeled " % Yield decrease per subsequent dS/m increase," reflect the loss of yield with each additional increase in the salt index of 1 dS/m. Figure 1 shows the effects of increasing salt index on the relative yield of sweet corn. No loss of sweet corn yield is observed until the salt index increases to the Salinity effect threshold. Yield then declines with a slope equal to the % yield decrease per subsequent dS/m increase. Clients using computer-based yield predictors may find these columns of benefit, due to ease of data entry.
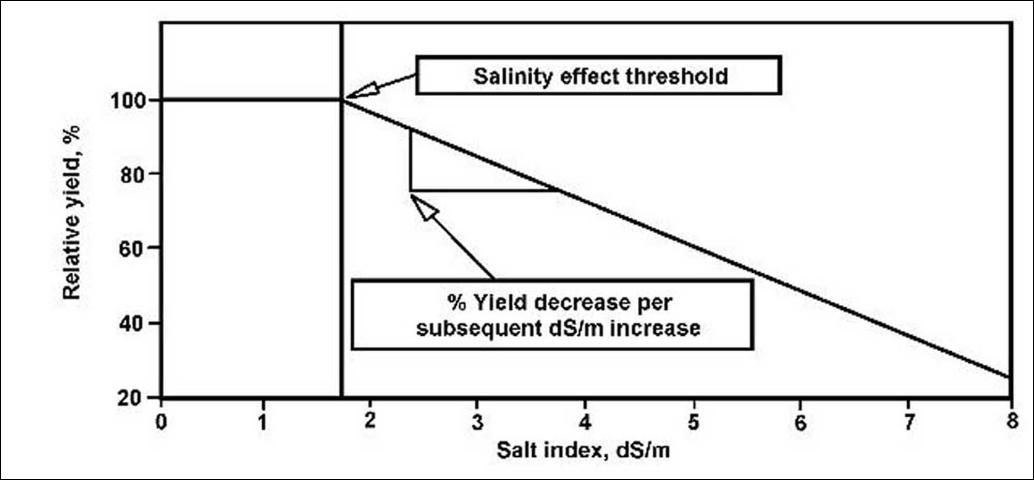
After calculating the salt index, Tables 1 through 4 may then be used according to this checklist:
- Find the plant(s) of concern in the tables.
- Reading across the top of the table, locate the "Salinity effect threshold (dS/m)" and "% Yield decrease per subsequent dS/m increase" columns.
- Read the column entries in the row corresponding to the selected plant.
- If the salinity effect threshold is greater than the measured salt index, then no salt damage to the crop is expected.
- If the salinity effect threshold is less than the measured salt index, then the potential for yield loss exists.
- To estimate potential yield loss at the measured salt index, use the following equation:
- Potential yield loss = (measured salt index - Salinity effect threshold) x %Yield decrease per subsequent dS/m increase.
For example, if the salt index of a soil is 2.4 and you have a bermudagrass lawn, refer to Table 4. The observed value of 2.4 is less than the Salinity effect threshold (6.9 dS/m). Therefore, salts are not expected to cause significant yield loss.
Using the same salt index of 2.4 dS/m for the production of clover (Table 1), the observed value (2.4) is greater than the Salinity effect threshold of 1.5. Therefore, salts will cause a loss of clover production. To calculate the potential yield loss at this level of salinity:
Potential yield loss = (2.4 dS/m - 1.5 dS/m) x 12% = 10.8
Thus, about 11% yield loss is expected due to salinity. In other words, expected yield is about 89% of the yield which could be obtained if salts were not present or were in lower amounts.
Checklist for Identifying Salt Problems
Water
- Measure the electrical conductivity (EC).
- If the EC is greater than 2.0 deciSiemens per meter, water must usually be mixed with another water source having lower EC, or not used for irrigation. An exception to this recommendation can be made for experienced water managers.
Soil
- Measure the EC of a 2:1 extract, beginning with air-dry soil.
- Convert the 2:1 reading to a salt index (multiply by 8).
- Use Tables 1, 2, 3, or 4 to determine if the measured salt index will affect selected crop(s).
- If salts will affect the selected crop(s):
- Provide drainage by ditching, tile or mole drains, etc.
- Leach the soil with high-quality water. The amount of irrigation water depends upon the depth of the rooting zone (volume of soil), the quality of water, and the method of application to the field. Using water with an EC less than 2.0 deciSiemens per meter is recommended. For sandy Florida soils, 2 to 3 inches of water should provide enough leaching to remove 90% of the salts from the upper 1.5 to 2 feet of soil.
Container Media
- Measure the EC of moist container media.
- Using the interpretations for potting media provided in Table 5, determine if the EC is High or Very High.
- If the EC is high enough that salts will affect the crop:
- Provide drainage to the container and add high quality irrigation water so that about 20% of the volume added leaches from the container.
- Repeat until EC returns to the normal range.
References
Bresler, E., B.L. McNeal and D.L. Carter. 1982. Saline and Sodic Soils, Springer-Verlag. Berlin, Germany. 236 pp.
Kidder, G. 1992. Determination of pH, soluble salts, nitrate, phosphorus, potassium, calcium, magnesium, sodium, and chloride in potting media (non-soil mixes) by saturation extraction. In S.J. Donohue (ed.) Southern Coop. Series Bull. No. 374. pp. 28-32.
Relative yields of selected field and forage crops at selected salt-index levels (Bresler et al. 1982).
Relative yields of selected vegetable crops at selected salt-index values (Bresler et al. 1982).
Relative yields of selected fruit crops at selected salt-index values (Bresler et al. 1982).
Relative yields of selected ornamentals at selected salt-index values (Bresler et al. 1982).