Objective
The objectives of this document are to provide readers with: 1) an overview of water clarity and how it is influenced by the presence of turbidity, suspended solids, and color; 2) an understanding of the importance of clarity from an ecological perspective; 3) a summary of methods for measuring each parameter; 4) the current state regulations for each in public waters of Florida; and 5) management opportunities to protect and improve water clarity. A glossary is provided at the end of this document for terms that are in bold in the text.
Introduction
Water clarity refers to the transparency or clearness of water. Turbidity is often used as a general term to describe the lack of transparency or "cloudiness" of water due to the presence of suspended and colloidal materials such as clay, silt, finely divided organic and inorganic matter, and plankton or other microscopic organisms. By definition, turbidity is caused primarily by suspension of particles smaller than 1 micron in diameter in the water column, while the term suspended solids refers to particles that typically range from 10 to 100 microns in diameter. For discussion purposes within this document, turbidity will refer to any of the suspended components that cause clouding of water. Visibility or clarity decreases as the turbidity increases in a given water body. The reduction in clarity is due to scattering of sunlight by suspended particles in the solution. In effect, the particles act as tiny mirrors that redirect incoming sunlight in other directions, thus reducing light penetration (Figure 1).
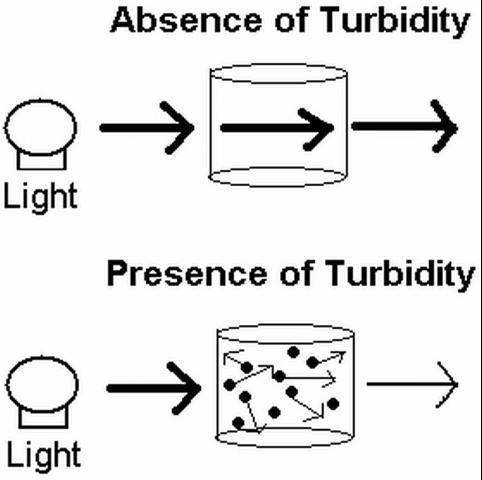
Credit: PCW
In addition to turbidity, water clarity is also influenced by water color. Pure water is transparent and colorless, allowing light from the sun or other sources to pass through. However, colored components in water absorb light energy preventing it from penetrating as deeply as in colorless water. In addition, certain wavelengths of light are reflected back out of the water, allowing us to see color.
Sources of Turbidity, Suspended Solids, and Color
Substances that reduce water clarity can originate from natural, developing, and developed areas within a watershed. In all cases, sand, silt, clay, and organic particles may be dislodged from land surfaces by rainfall and carried by overland flow. The risk of contributing turbidity to aquatic systems is highest when land surfaces are bare and rainfall events occur. Turbidity may also result from the formation of algal blooms. Algal blooms form in response to nutrient import into the aquatic system and from nutrients released during the decomposition of aquatic plants and other organisms. In many aquatic systems, water clarity is determined by the abundance of suspended algae. Eutrophic systems (containing high nutrient concentrations) support large algal populations, which reduce the clarity of the water and increase its color. Under these conditions, the majority of primary production is due to algal photosynthesis and is restricted to the upper layers of water.
While turbidity does occur naturally and from natural sources, turbidity originating from anthropogenic activities can be of a much higher magnitude, especially since waterfront areas tend to be highly developed. Among these activities, construction and agricultural production tend to disturb land surfaces, potentially contributing soil particles as well as nutrients to aquatic systems. Likewise, lawn and landscape fertilization and aquatic weed control activities tend to add nutrients and plant materials that can contribute nutrients, color, and organic matter to water bodies. Dredging of water bodies resuspends fine particulates, increasing turbidity. Occasionally sewage treatment plants may contribute organic materials and nutrients to water bodies during sewage bypass periods or when infrastructure fails.
Color in water may result from a variety of sources including natural metallic ions (iron and manganese), humus and peat materials, plankton, weeds, and industrial wastes. Metallic ions such as iron and manganese typically impart a reddish-brown color to water. Tannins and dissolved organic carbon, a by-product of the degradation of plants and other organisms, usually impart a brown to black color to water. Some living plants such as parrotfeather (Myriophyllum aquaticum) also release colored organic compounds into the water column.
Ecological Significance
Water clarity is important in determining the depth-of-penetration of sunlight within a given water body. Light penetration is especially important for submerged aquatic plants such as seagrasses, American Tapegrass, and hornworts. These plants depend on light interception and absorption for photosynthesis. Enough light must be absorbed by the plant for photosynthesis to result in a net increase in biomass in order for the plant to grow and reproduce. As the amount of light decreases, the plant reaches a point where the products of photosynthesis are equal to the products consumed by respiration. At this point, called the light compensation point, no net growth occurs and CO2 uptake is equal to O2 release. As light levels further decrease, the plant begins to consume more photosynthate for respiration than can be produced by photosynthesis.
Under these conditions, the plants begin to die off, reducing their biomass to a level that can be sustained at the lower level of photosynthesis. Further reductions in light levels result in death of the plant. As a result, plant growth in waters containing much turbidity and color is typically restricted to shallow depths, whereas plant growth is less restricted in non- or less-turbid/colored water. Submerged aquatic vegetation is a very important component of aquatic ecosystems. It provides food, shelter, and protection for many different aquatic invertebrate and vertebrate species. Declines in this habitat will indirectly affect populations of species that depend on it. Restrictions in light penetration depth due to turbidity and color are especially important in estuarine systems that support seagrasses. Seagrasses typically require much more light than terrestrial plants and algae for photosynthesis, possibly due to inefficiencies in photosynthesis and toxic sulfides in sediments within the marine environment (Kemp 2000).
In addition to impacting submersed plant communities and productivity, suspended solids may clog the filtering apparatus and digestive tracts of planktonic organisms as well as irritate and/or injure the gills of fish (Schueler 1997). Excessive amounts of turbidity can also affect migration patterns for sensitive species, as well as feeding-activity rates. As a result of these and other individual effects, turbidity can induce many changes to the composition of aquatic communities, resulting in reductions in the overall numbers of submersed aquatic macrophytes and aquatic invertebrates, and ultimately leading to declines in some fish populations due to reduced food and habitat resources. Sensitive or threatened fish species may be lost when turbidity exceeds 100 NTU (Schueler 1997). Fish communities in areas subject to high amounts of suspended solids typically shift toward more sediment-tolerant species (Schueler 1997).
Dissolved oxygen (DO) levels within affected water bodies may also decline when turbidity is caused by organic particles due to natural degradation of the materials by microbial populations. Many microorganisms use organic carbon as a source of energy for respiration and consume oxygen in the process. Additionally, nutrients often leach from decaying organic materials into surface water. These nutrients may contribute to over stimulation of algal growth and production (algal blooms). Increased levels of algae are often associated with very low concentrations of DO during dark periods (especially at dawn) due to respiration, often resulting in extensive fish kills.
Anthropogenic Significance
Turbidity, suspended solids, and color can impact the suitability of water bodies for recreational activities. Because they reduce water clarity, potentially dangerous obstructions such as rocks, stumps, sand bars, and logs may not be visible to boaters and swimmers. Additionally, turbidity and suspended solids may be a contributing factor to waterborne disease outbreaks due to microorganisms (bacteria, viruses, and protozoans) possibly associated with the organic fractions.
Turbid water may not be suitable for use in a variety of industrial applications, ranging from manufacturing to agriculture. In the manufacturing industries, suspended solids may clog or cause excessive wear to pipes and machinery. Turbid water may also not be suitable for agricultural microsprinkler irrigation because of the high likelihood of quickly clogging filters, tubing, and emitters. Excessive turbidity can also decrease the efficacy of herbicides used for aquatic weed control due to sorption. In this case, inadequate weed control may result. Use of turbid water for tank-mixing pesticides may also result in lower pesticide efficacy and increased wear on pumps and nozzles. This generally depends on the solubility and sorptive properties of the particular pesticide and on the composition of the turbidity-causing agent.
Measurement
Water clarity and turbidity are usually measured in the field using a Secchi disk and turbidimeter, respectively. Suspended solids are measured by filtration in the lab. Water color can be measured in the field with portable kits, or within the lab. For measurements requiring sampling, always collect samples in clean containers. Make measurements shortly after the samples are collected because the character of the samples will change over time due to physical and biological processes that occur during storage. The highlights for each method are described in the following sections. See referenced methods for more details.
Clarity
The Secchi disk is a useful tool for measuring water clarity (Figure 2). This disk (20cm dia.) is marked with alternating quarter sections of black and white (for freshwater) or is solid white (for salt water). It is lowered through the water column until it is no longer visible. The depth from the surface to the point where it disappears is called the Secchi depth. This depth is a surrogate measurement for the light compensation point for submerged aquatic plants. Secchi depths for lakes of differing trophic status are listed in Table 1. For comparisons, the Secchi depth of a water body filled with distilled water would be greater than 136.6 meters (120 feet). Secchi depth measurements are usually only applicable to relatively still water bodies such as slow-flowing streams/canals, ponds, lakes, estuaries, and the ocean. Routine Secchi measurements can be very useful for identifying water clarity trends.

Credit: Forestry Suppliers, Inc. (http://www.forestry-suppliers.com)
Turbidity
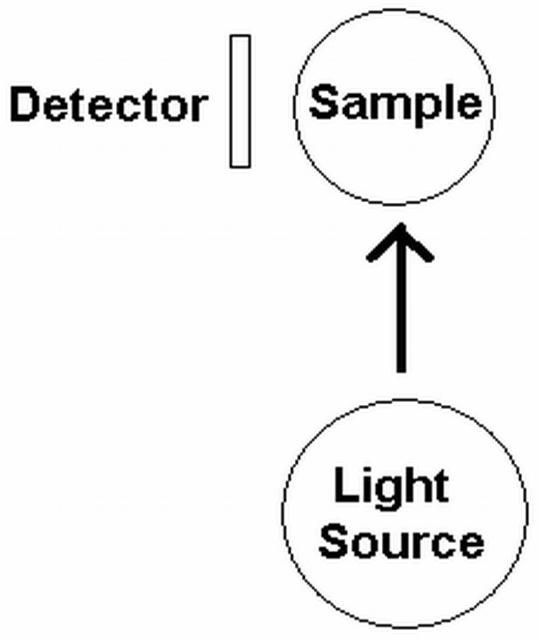
Turbidity is commonly measured in Nephelometric Turbidity Units (NTU) using an instrument referred to as a turbidimeter. Turbidimeters with scattered-light detectors located at 90 degrees to the incident light beam are called nephelometers (Figure 3 and 4). This instrument measures the amount of light scattering that occurs within a given water sample by shining a bright light on one side of the sample and measuring the amount redirected to the detector located 90 degrees to the incident light (direction of the incoming light). Scattering from the water sample is measured relative to the amount of light scattered by a reference solution (a solution that will cause a known amount of light scattering). The scattering of light increases as the amount of suspended materials in the water increases.

Credit: Orbeco-Hellige, Inc. (http://www.orbeco-hellige.com)
Credit: PCW
Nephelometers are specified as the standard instrument for measurement of low turbidities because responses are relatively unaffected by small differences in instrument design parameters. Even though different instrument makes and models will vary slightly in response, poor measurement technique can have a greater effect on measurement error than small differences in instrument design. Consult Standard Methods for Examination of Water and Wastewater for more details if needed.
Tips for Measurement
- Always follow manufacturer instructions for measuring turbidity with a given instrument.
- Measure turbidity immediately. Particle flocculation and sedimentation will occur over time and will change the original sample characteristics.
- Do not dilute samples. Dilution water may dissolve constituents that originally were particulate.
- Remove air bubbles from samples before measuring. Do not do this by letting the samples stand for some time period because sample characteristics may also change with time.
- If the sample is cooler than the measurement environment, some condensation may occur on the outside of the measurement container. If this occurs, wipe off the condensation before making measurements. The condensation will reduce the amount of light reaching the sample and detector.
- Calibrate the instrument with a least one standard in the range used to ensure accurate readings.
- Before reading, samples should be gently agitated. Once all air bubbles disappear, pour the sample into the measurement container and place in the instrument for reading.
Detection Limits
An acceptable turbidimeter should be able to discriminate turbidity differences of 0.02 NTU, with a range of 0 to 40 NTU.
Interferences
Coarse debris that rapidly settles, dirty glassware, presence of air bubbles, and surface vibrations will alter turbidity readings. Water color due to dissolved substances that absorb light causes measured turbidities to be lower than actual.
Total Suspended Solids
Measurement of suspended solids requires the use of pre-rinsed, pre-dried glass fiber filters with an effective pore size of 0.45 µm. The weight of prepared filters should be noted before processing the sample. A well-mixed sample of water is filtered through a prepared glass fiber filter, leaving the residual suspended solids on the filter. This residue and the filter are then dried to constant weight at 103ºC–105ºC in an oven. Once dry, the filter containing the retained residues is weighed again. This method does not discriminate between organic and inorganic constituents.
The concentration of suspended solids is calculated as follows:
Suspended Solid residue (mg/L) = [(Weight (mg) of filter + residue) – (Weight (mg) of filter alone)] / Volume of sample filtered (L)
Color
There are a variety of methods for measuring water color that range from simple comparisons to more complicated analyses using specialized equipment. The visual comparison method is the simplest and is summarized below. Consult Standard Methods for Examination of Water and Wastewater for more detailed information.
The visual comparison method for measuring color is based on visually comparing the color of the sample to standardized colors produced by standardized chemical solutions or standardized colors embedded in special property-calibrated glass color disks. The more accepted standard method utilizes a platinum-cobalt solution as the standard and is called the platinum-cobalt method. Using this method, the color of the water sample is determined relative to the color produced by dissolving specific amounts of platinum (as chloroplatinate ion) in one liter of distilled water. The standard chloroplatinate solution can be made by dissolving 1.246 g potassium chloroplatinate and 1 g crystalline cobaltous chloride in distilled water containing 100 mL of concentrated hydrochloric acid. This solution is diluted to 1000 mL with distilled water, resulting in a standard solution that is equivalent to 500 color units. Aliquots of this solution are then diluted in 50 mL of distilled water to generate a series of color ranges for comparison with the sample. The color of the standard solution closest to the sample color is the estimated color of the sample. Instead of using the prepared chloroplatinate dilution series for standards, the more commonly used equipment uses a glass disc with the color standards embedded within it. The colors embedded within the disc are based on the chloroplatinate dilution series.
For color measurements, the sample is placed in a specialized glass tube. The tube is then inserted into an apparatus (Figure 5) that directs light upward through the bottom of the tube. In the case of the liquid chloroplatinate standards, one tube is filled with the standard solutions that are changed until one matches the color of the sample. If using the color-embedded glass discs, the color disc above the distilled water-filled tube is turned until the color matches that of the sample for color determination.

Credit: Orbeco-Hellige, Inc. (http://www.orbeco-hellige.com)
Interferences
The presence of any turbidity in the sample can cause the apparent color to be significantly higher than the true color. Remove turbidity by centrifugation or by filtration before approximating the true color.
The color value of a water sample can also be very dependent on the pH of the sample. It usually increases as the pH of the water increases. For this reason, always record and report the pH value of the water sample when color measurements are taken to aid in comparisons to past, future, or present conditions. If resources are available, determine the color value at various pH values to aid with comparisons among different laboratories if needed.
Applicability
The visual comparison method is useful for measuring the color of potable water and of water in which color is due to naturally occurring materials. This method is not applicable for measurements of highly colored industrial waste waters.
Florida Surface Water Quality Criteria
It is important to recognize that the most desirable water clarity for one water body may not be the same as for others. The desired clarity ultimately depends on the desired use for the water body.
The Florida Department of Environmental Protection (FDEP) is charged with protecting water quality within state-owned surface water bodies for their respective designated uses. To accomplish this goal, FDEP has defined criteria for transparency and turbidity in state surface water bodies. Table 2 lists the designated uses for each surface water classification group and the FDEP's criteria. Water quality restrictions are greater for numerically lower classes of water (i.e., I > II > III > IV > V). Water flowing from a numerically higher class of water (for example, class IV) into a more restrictive class (i.e., a numerically lower class) may be required to meet standards for the lower class.
Management Opportunities for Protecting and Improving Water Clarity
Stabilize bare soils. Stabilize bare soils and canal, ditch, and other water body banks by encouraging coverage by vegetation. Vegetation types selected should be adapted to local conditions and should provide maximum stabilization by roots and foliage. Vegetative buffer strips could also serve to reduce the erosion of soil particles. In areas subject to high water velocities, protect banks with hard materials such as rip-rap, concrete, headwalls, or other materials that buffer turbulence. In construction areas, use silt and turbidity fencing materials, as well as hay bales or other filtering materials.
Water discharge control structures. Choose structures that discharge over the top of water control surfaces as opposed to those that discharge from the bottom to facilitate settling of larger particles.
Side bank slope. Construct ditches, canals, and ponds/lakes with side-slopes consistent with soil types. Refer to engineering tables in local county soil surveys for information on preferred side slopes for specific soil types.
Aquatic plant management and dredge timing. Limit herbicide applications and dredging activities to periods of no rainfall or when no flow occurs from the work area. Prevent discharge of mowed plant materials from ditch banks into water systems.
Encourage growth of submerged aquatic plants. Coverage of 50% or more of the bottom surface area can drastically reduce turbidity from algal blooms.
References and Suggested Readings
American Public Health Association (APHA), American Water Works Association (AWWA), and the Water Environmental Federation (WEF). Standard Methods for Examinations of Water and Wastewater. United Book Press, Inc. Baltimore, Maryland.
Bachmann, M., M. Hoyer, and D. Canfield, Jr. 1999. Living at the Lake. Gainesville: University of Florida Institute of Food and Agricultural Sciences.
Burton, G. A., Jr., and R.E. Pitt. 2002. Storm Effects Handbook: A Toolbox for Watershed Managers, Scientist, and Engineers. Lewis Publishers, Boca Raton, FL.
Florida LAKEWATCH. 2001. A Beginner's Guide to Water Management—Water Clarity. CIR103. https://edis.ifas.ufl.edu/fa080 Gainesville: University of Florida Institute of Food and Agricultural Sciences.
Kemp, W. M. 2000. "Seagrass Ecology and Management: An Introduction." In Seagrasses: Monitoring, Ecology, Physiology, and Management. Bortone, S.A. (ed). CRC Press, Boca Raton, FL. pp. 1–6.
Schueler, T. (Ed.) 1997. "Comparison of Forest, Urban and Agricultural Streams in North Carolina." Watershed Protect. Tech. 2(4): 503–506. June 1997.
USDA/NRCS National Engineering Handbook, Section 16, Drainage of Agricultural Land; Chapter 5: Open Ditches for Drainage – Design, Construction, and Maintenance. https://directives.sc.egov.usda.gov/OpenNonWebContent.aspx?content=18366.wba
US EPA. 1971. EPA Method #:160.2: Residue, Non-Filterable (Gravimetric, Dried at 103-105 C). Methods for the Chemical Analysis of Water and Wastes (MCAWW) (EPA/600/4-79/020).
Wilber, C. G. 1983. Turbidity in the Aquatic Environment: An Environmental Factor in Fresh and Oceanic Waters. Charles C. Thomas Publishers, Springfield, IL.
Glossary
Invertebrate—Animals possessing no distinct cartilaginous or bony axial endoskeleton.
Light compensation point—The light intensity where photosynthesis and respiration are equal. At this point, there is no net change in the concentration of O2 or CO2.
Macrophyte—Large aquatic plant.
Photosynthate—Energy-rich, carbonaceous compounds formed by photosynthesis.
Photosynthesis—The assimilation of carbon dioxide by green plants and by pigmented, photosynthetically active prokaryotes, and its conversion into carbohydrate, using energy from the sun. This conversion can be described by the following equation:
6CO2 + 12 H2O +energy ? C6H12O6 + 6O2 + 6H2O
Primary productivity—Refers to the rate at which energy from the sun is stored (via photosynthesis) in an ecological community or group of communities.
Respiration—Metabolic process in which stored energy is released with the oxidation of reduced organic carbon compounds. The ultimate by-products of respiration are often carbon dioxide (CO2) and water (H2O).
Sorptive properties—Properties that influence adsorption or absorption of one substance by another.
Transparency—As defined by the Florida Department of Environmental Protection, transparency is the depth to the compensation point for photosynthetic activity.
Trophic status—A term that relates the nutritional status and primary production status of a given water system. Plant growth increases as nutritional status increases.
Lake trophic status and associated nitrogen, phosphorus, chlorophyll a, and Secchi depth measurements. Adapted from Bachmann et al. (1999).
Designated water-use groups and turbidity and color criteria for surface waters of the state of Florida. Source: Florida Department of Environmental Protection—https://www.flrules.org/gateway/RuleNo.asp?title=SURFACE%20WATER%20QUALITY%20STANDARDS&ID=62-302.530