This publication is intended to provide biological information of the soil fungal taxa Mortierella elongata. M. elongata is one of the most ubiquitous fungi living in the soil. The preliminary data generated from our recent studies indicated that M. elongata is one of the most abundant taxa (>0.5% of total soil fungi) living in the agriculture soils collected from north Florida, including pastures, cotton fields, and peanut fields. Our recent studies showed that M. elongata can perform plant-growth promotion across different types of crops, including bahiagrass, corn, tomato, squash, and watermelon (Zhang et al. 2020). Compared to the well-known species that have plant-growth-promotion abilities (e.g., mycorrhizal fungi, Trichoderma), growers, agents, and stakeholders are not familiar with "Mortierella" despite the important role these fungal taxa play in promoting the growth of their crops. Only very recently, growing studies started to focus on the function of this basal fungus. It is important to transfer the updated knowledge generated from research to Extension, given that this fungus might have potential to serve as a bioindicator of soil health or as a bio-N-fertilizer in the future. This publication provides a brief overview of Mortierella from biological, taxonomical, ecological, and functional perspectives to help readers learn the biology and potential modes of action of this fungus.
Summary
Mortierella is a widespread genus of fungal generalists that are detected as root endophytes and saprophytes in many natural and agricultural habitats, including forests, pastures, and croplands. Mortierella elongata has been observed to promote the growth of many plant species, including cottonwood, pine, oak, grass, tomato, corn, and Arabidopsis spp. The growth-promotion ability of M. elongata is plant-species independent. This evidence suggests that M. elongata is a potential bioindicator and biocontrol agent for crop production and soil health. The taxonomy, biology, and distribution of M. elongata and its potential modes of action on plant-growth promotion are discussed in this article.
Taxonomy
M. elongata (=Linnemannia elongata), is one of the most common representatives of genus Mortierella, a genus that contains more than 100 validated species (Ainsworth 2008; Vandepol et al 2022). Mortierella, within the family Mortierellaceae of the order Mortierellales, belongs to Mucoromycota, an early-diverging phylum of fungi. Mucoromycota is comprised of Glomeromycotina (arbuscular mycorrhizal fungi), Mortierellomycotina, and Mucoromycotina (Bidartondo et al. 2011; Spatafora et al. 2016).
Biology
Of over 100 recognized species of Mortierella, only M. wolfii is reported to be a pathogenic species as a cause of abortion in cows (Davies et al. 2010). Although the ecological functions of most Mortierella spp. are unknown, a recent study with a few Mortierella spp. suggest their promising influences on plant-growth promotion, lipid production, and chitin decomposition.
Mortierella spp., the filamentous fungi (fungi that form threadlike structures known as hyphae), are thought to include the first terrestrial fungi to evolve distinct fruiting bodies. Most Mortierella spp., including M. elongata, are culturable and can grow fast under different culture conditions. In general, the mycelia of M. elongata in culture are white, with a rosette-like surface appearance and no pigment (Figure 1A). The culture grows well at room temperature (between 20°C and 25°C). The zygospore, sporangiophores, sporangia, and chlamydospores may be present (Figure 2). The formation of different spore types is media dependent. For example, SAB-sucrose agar, hay extract agar, and 2% malt extract agar (MEA) are more suitable for zygospore formation of the heterothallic strains (Gams et al. 1972). The SEA medium (but not MEA) is more suitable for the sporulation of sporangia. Chlamydospores can be produced on MEA (Gams 1976).
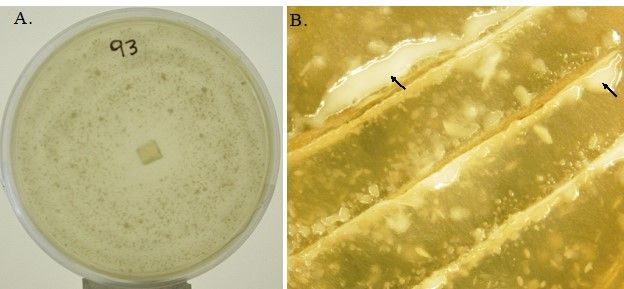
Credit: Liao et al. (2019)
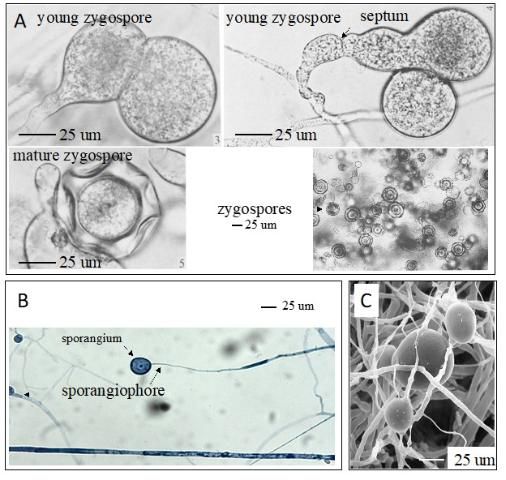
Credit: A: Gams et al. (1972), RightsLink License Number 4900820129589; B: Khalid Hameed; C: ZyGoLife Research Consortium
M. elongata metabolism in culture is mostly based on utilization of simple carbon (e.g., D-glucose, D-trehalose and D-mannose) (Uehling et al. 2017). In nature, M. elongata can utilize N-acetyl glucosamine (chitin monomer) as carbon and nitrogen sources and serve as the most important chitin decomposers in the soil (Gray and Baxby 1968). Many Mortierella species have been validated to be excellent candidates for lipid and biofuel production (e.g., linoleic acid, α-linolenic acid, arachidonic acid and docosahexaenoic acid) (Kendrick and Ratledge 1992; Meeuwse et al. 2012; Kikukawa et al. 2018; Vadivelan and Venkateswaran 2014; Du et al 2018). M. elongata is able to produce these lipids in culture (Figure 1B) and in the rhizosphere (Liao et al. 2019). Most of these polyunsaturated fatty acids are essential components that mediate vital biological functions of organisms. While coculturing with plants, M. elongata forms a biofilm on plant roots, indicating their ability to directly interact with root cells as the fungal endophyte (Figure 3).
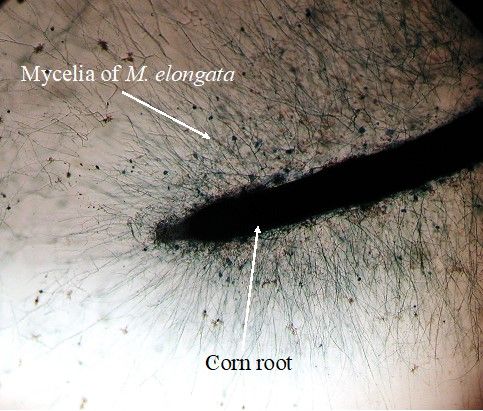
Credit: Liao et al. (2019)
Distribution
M. elongata is one of the most prevalent species of soil fungi. It can grow saprotrophically in the soil and is also isolated as an endophyte from healthy plant roots (Liao et al. 2019; Bonito et al. 2016). M. elongata adapts very well to a wide range of environmental conditions. For example, M. elongata has been reported or isolated from fell-field soils in the Antarctic (Weinstein et al. 2000), tundra soils in Alaska (Gams et al. 1972), alpine forests in Norway (Robinson 2001), arid agricultural regions of India (Niu et al. 2018), coastal regions of Mexico, long-term corn fields in China (Li et al. 2018), and cottonwood, forage, and cotton fields in North America (Liao et al. 2019) (Figure 4). M. elongata grows well in a range of soil types from pH levels of 4 to 7 and prefers litter and upper organic soil horizons. The DNA sequence-based methods aid in rapid quantification and identification of soil microorganisms in situ. Using this approach, Mortierella spp. (including M. elongata) were detected in high abundance (0.3%–0.8% of total soil fungi) in the soils in forests, pastures, and agriculture fields (Figure 4).
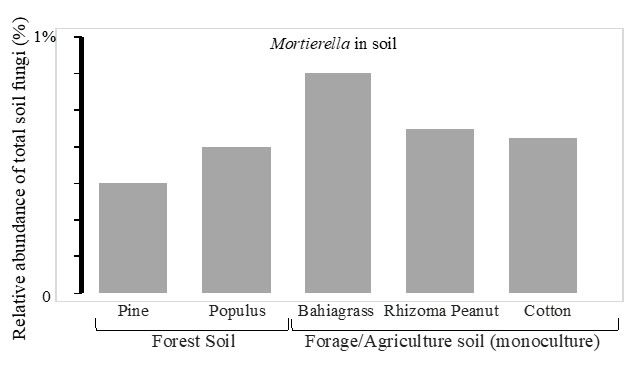
Credit: Liao, unpublished data
Potential Modes of Action of Mortierella elongata on Plant-Growth Promotion
The beneficial associations between Mortierella and plants have only recently begun to be understood. M. elongata enhanced the biomass of roots and aboveground tissues, enhanced leaf expansion, and increased the amount of chloroplasts in cottonwood (Liao et al. 2019), pine (Figure 5), corn, watermelon, tomato, and squash (Li et al. 2018; Zang et al 2020). M. elongata in the soil can increase the activities of soil degradation enzymes of C and P (e.g., phosphatase and beta-glucosidase) (Li et al. 2018). Some other species of Mortierella also have been reported to be beneficial fungi of plants. For example, Mortierella hyalina enhanced biomass of cress (Arabidopsis) (Johnson et al. 2018). Mortierella alpina enhanced the stress tolerance of saffron crocus (Crocus sativus) by promoting the production of tetraterpenoid-associated phytohormones (Wani et al. 2017). Overall, these recent findings suggest that genus Mortierella plays an important role in soil and plant health. The underlying mechanisms of Mortierella associated with plant-growth promotion are still largely unknown. The potential modes of action of Mortierella on plants are only starting to be uncovered, possibly including (1) enhancing plant growth-hormone production (e.g., IAA, GA, and ABA) (Liao et al. 2019; Li et al. 2018); (2) suppressing plant defense responses (Johnson et al. 2018; Liao et al. 2019); and (3) mediating plant lipid pathways (Liao et al. 2019).
![Figure 5 Figure 5. Growth enhancement of loblolly pine (Pinus taeda) in response to inoculation of M. elongata (Isolate PMI93). After inoculation, seedlings of P. taeda were grown in sterile sand or natural soil systems (30% soil collected from P. taeda forest, Durham, NC, mixed with 70% sterile sand [w/w]) for 10 months.](/image/SS679/D8wunnaqb7/Dks2um20y1/Dks2um20y1-2048.webp)
Credit: Hui-Ling Liao, UF/IFAS
Mortierella elongata–Bacteria Endophyte Interactions
M. elongata living in the soil harbor diverse bacteria taxa inside their hyphae cells (Sato et al. 2010; Uehling et al. 2017; Ohshima et al. 2016). These bacteria can alter the morphology and metabolism of their host fungi and at the same time rely on their fungal partner for the support of amino acids. It is not known if such a fungal-bacterial symbiont system might be associated with the growth-promotion activities of M. elongata.
References
Ainsworth, G. C. 2008. Ainsworth & Bisby's Dictionary of the Fungi. CABI.
Bidartondo, M. I., D. J. Read, and J. M. Trappe. 2011. "The Dawn of Symbiosis between Plants and Fungi." Biology Letters 7 (4): 574–577. https://doi.org/10.1098/rsbl.2010.1203
Bonito, G., K. Hameed, R. Ventura, J. Krishnan, C. W. Schadt, and R. Vilgalys. 2016. "Isolating a Functionally Relevant Guild of Fungi from the Root Microbiome of Populus." Fungal Ecol. 22:35–42. https://doi.org/10.1016/j.funeco.2016.04.007
Davies, J. L., M. Ngeleka, and G. A. Wobeser. 2010. "Systemic Infection with Mortierella wolfii following Abortion in a Cow." Can. Vet. J. 51:1391.
Du, ZY., Alvaro, J., Hyden, B. et al. 2018. “Enhancing oil production and harvest by combining the marine alga Nannochloropsis oceanica and the oleaginous fungus Mortierella elongata.” Biotechnol Biofuels 11:174. https://doi.org/10.1186/s13068-018-1172-2
Gams, W. 1976. "Some New or Noteworthy Species of Mortierella." Persoonia-Molecular Phylogeny and Evolution of Fungi. 9:111–140.
Gams, W., C.-Y. Chien, and K. H. Domsch. 1972. "Zygospore Formation by the Heterothallic Mortierella elongata and a Related Homothallic Species, M. epigama sp.nov." Trans. Br. Mycol. Soc. 58:5–13, IN1–IN2. https://doi.org/10.1016/S0007-1536(72)80065-2
Gray, T. R. G., and P. Baxby. 1968. "Chitin Decomposition in Soil: II. The Ecology of Chitinoclastic Micro-organisms in Forest Soil." Trans. Br. Mycol. Soc. 51:293–309. https://doi.org/10.1016/S0007-1536(68)80064-6
Johnson, J. M., A. Ludwig, A. Furch, A. Mithöfer, S. S. Scholz, M. Reichelt, and R. Oelmüller. 2018. "The Beneficial Root-Colonizing Fungus Mortierella hyalina Promotes the Aerial Growth of Arabidopsis and Activates Calcium-Dependent Responses That Restrict Alternaria brassicae–Induced Disease Development in Roots." Mol. Plant. Microbe. Interact. 32 (3): 351–363. https://doi.org/10.1094/MPMI-05-18-0115-R
Kendrick, A., and C. Ratledge. 1992. "Lipids of Selected Molds Grown for Production of n-3 and n-6 Polyunsaturated Fatty Acids." Lipids 27:15–20. https://doi.org/10.1007/BF02537052
Kikukawa, H., E. Sakuradani, A. Ando, S. Shimizu, and J. Ogawa. 2018. "Arachidonic Acid Production by the Oleaginous Fungus Mortierella alpina 1S-4: A Review." J. Advert. Res. 11:15–22. https://doi.org/10.1016/j.jare.2018.02.003
Li, F., L. Chen, M. Redmile-Gordon, J. Zhang, C. Zhang, Q. Ning, and W. Li. 2018. "Mortierella elongata's Roles in Organic Agriculture and Crop Growth Promotion in a Mineral Soil." Land Degrad. Dev. 29:1642–1651. https://doi.org/10.1002/ldr.2965
Liao, H.-L., G. Bonito, J. A. Rojas, K. Hameed, S. Wu, C. W. Schadt, J. L. Labbe, G. Tuskan, F. M. Martin, I. V. Grigoriev, and R. Vilgalys. 2019. "Fungal Endophytes of Populus trichocarpa Alter Host Phenotype, Gene Expression and Rhizobiome Composition." Mol. Plant. Microbe. Interact. 32 (7): 853–864. https://doi.org/10.1094/MPMI-05-18-0133-R
Meeuwse, P., P. Akbari, J. Tramper, and A. Rinzema. 2012. "Modeling Growth, Lipid Accumulation and Lipid Turnover in Submerged Batch Cultures of Umbelopsis isabellina." Bioprocess Biosyst. Eng. 35:591–603. https://doi.org/10.1007/s00449-011-0632-x
Niu, Y., L. D. Bainard, W. E. May, Z. Hossain, C. Hamel, and Y. Gan. 2018. "Intensified Pulse Rotations Buildup Pea Rhizosphere Pathogens in Cereal and Pulse Based Cropping Systems." Front. Microbiol. 9:1909. https://doi.org/10.3389/fmicb.2018.01909
Ohshima, S., Y. Sato, R. Fujimura, Y. Takashima, M. Hamada, T. Nishizawa, K. Narisawa, and H. Ohta. 2016. "Mycoavidus cysteinexigens gen. nov., sp. nov., an Endohyphal Bacterium Isolated from a Soil Isolate of the Fungus Mortierella elongata." Int. J. Syst. Evol. Microbiol. 66:2052–2057. https://doi.org/10.1099/ijsem.0.000990
Robinson, C. H. 2001. "Cold Adaptation in Arctic and Antarctic Fungi." New Phytol. 151:341–353. https://doi.org/10.1046/j.1469-8137.2001.00177.x
Sato, Y., K. Narisawa, K. Tsuruta, M. Umezu, T. Nishizawa, K. Tanaka, K. Yamaguchi, M. Komatsuzaki, and H. Ohta. 2010. "Detection of Betaproteobacteria inside the Mycelium of the Fungus Mortierella elongata." Microbes Environ. 25:321–324. https://doi.org/10.1264/jsme2.ME10134
Spatafora, J. W., Y. Chang, G. L. Benny, K. Lazarus, M. E. Smith, M. L. Berbee, G. Bonito, N. Corradi, I. Grigoriev, A. Gryganskyi, T. Y. James, K. O'Donnell, T. N. Taylor, J. Uehling, R. Vilgalys, M. White, and J. E. Stajich. 2016. "Zygomycete Genealogy of Life (ZyGoLife): A Phylum-Level Phylogenetic Classification of Zygomycete Fungi Based on Genome-Scale Data." Mycologia 108 (5): 1028–1046. https://doi.org/10.3852/16-042
Uehling, J., A. Gryganskyi, K. Hameed, T. Tschaplinski, P. K. Misztal, S. Wu, A. Desirò, N. Vande Pol, Z. Du, A. Zienkiewicz, K. Zienkiewicz, E. Morin, E. Tisserant, R. Splivallo, M. Hainaut, B. Henrissat, R. Ohm, A. Kuo, J. Yan, A. Lipzen, M. Nolan, K. LaButti, K. Barry, A. H. Goldstein, J. Labbé, C. Schadt, G. Tuskan, I. Grigoriev, F. Martin, R. Vilgalys, and G. Bonito. 2017. "Comparative Genomics of Mortierella elongata and Its Bacterial Endosymbiont Mycoavidus cysteinexigens." Environ. Microbiol. 19:2964–2983. https://doi.org/10.1111/1462-2920.13669
Vadivelan, G., and G. Venkateswaran. 2014. "Production and Enhancement of Omega-3 Fatty Acid from Mortierella alpina CFR-GV15: Its Food and Therapeutic Application." Biomed Res. Int. 2014:657414. https://doi.org/10.1155/2014/657414
Vandepol, N., Liber, J., Yocca, A., Matlock, J., Edger, P., and Bonito, G. 2022. “Linnemannia elongata (Mortierellaceae) stimulates Arabidopsis thaliana aerial growth and responses to auxin, ethylene, and reactive oxygen species”. PLoS One. 2022:17(4):e0261908. https://doi: 10.1371/journal.pone.0261908
Wani, Z. A., A. Kumar, P. Sultan, K. Bindu, S. Riyaz-Ul-Hassan, and N. Ashraf. 2017. "Mortierella alpina CS10E4, an Oleaginous Fungal Endophyte of Crocus sativus L. Enhances Apocarotenoid Biosynthesis and Stress Tolerance in the Host Plant." Sci. Rep. 7:8598. https://doi.org/10.1038/s41598-017-08974-z
Weinstein, R. N., P. O. Montiel, and K. Johnstone. 2000. "Influence of Growth Temperature on Lipid and Soluble Carbohydrate Synthesis by Fungi Isolated from Fellfield Soil in the Maritime Antarctic." Mycologia 92:222–229. https://doi.org/10.2307/3761554
Zhang, K., G. Bonito, C.-M. Hsu, K. Hameed, R. Vilgalys, and H.-L. Liao. 2020. "Mortierella elongata Increases Plant Biomass among Non-leguminous Crop Species." Agronomy 10 (5): 754. https://doi.org/10.3390/agronomy10050754