Introduction
Florida seagrass meadows support an array of organisms (e.g., algae, invertebrates, fish, marine mammals, and sea turtles) and provide many benefits to humans (e.g., shoreline stabilization, water filtration, and carbon sequestration). Unfortunately, seagrass populations are in decline due to several threats, including eutrophication, climate change, and coastal development. Species diversity and genetic diversity increase seagrass ecosystem functions (i.e., physical, chemical, and biological processes) and stability (i.e., ability to maintain equilibrium post-disturbance). Therefore, incorporating diversity into restoration efforts may increase restoration success. In this paper, we review the importance of seagrass, major threats to seagrass, and ways in which seagrass species diversity and genetic diversity can positively impact seagrass management and restoration. This publication is intended for individuals interested in seagrass management, including Florida community groups and conservation/management agencies.
Importance of Seagrasses
Seagrasses are valuable to humans. As an ecosystem engineer, seagrass modifies its environment and acts as the base of the ecosystem. Seagrass biomass changes water flow and nutrient cycling, and provides structure which results in carbon sequestration, water filtration, habitat provisioning, erosion control, and support for tourism and fisheries (Figure 1).
Credit: This figure was created by the Integration and Application Network (ian.umces.edu/media-library)
Seagrasses play a major role in coastal nutrient cycles, storing carbon and improving water quality. Organic carbon, nitrogen, and phosphorus are all stored in seagrass biomass. Furthermore, these elements are buried in seagrass sediment at high rates. Low oxygen levels in seagrass sediments result in slow decomposition and the potential for long-term storage. This carbon storage can help to mitigate climate change because carbon stored in seagrass sediments is not being released in the atmosphere as a greenhouse gas. By increasing sediment organic matter and releasing small amounts of oxygen, seagrasses also promote denitrification, which is a process where microbes convert nitrogen from nitrate to gaseous nitrogen. The removal of nitrogen from the water via denitrification can sometimes limit the growth of algae and phytoplankton when it is nitrogen limited, thereby potentially lessening the impacts of harmful algal blooms (Flindt et al. 1999). Seagrasses can also help oxygenate the water column through photosynthesis which supports diverse animal communities.
Seagrass leaves and roots provide structure and habitat for epiphytic algae, invertebrates (e.g., shrimp and scallops), small fish, larger predatory fish, and larger herbivores (e.g., sea turtles and manatees). Smaller organisms, such as algae or invertebrates, rely on seagrass beds for habitat and protection from predators. Many fish species rely on seagrass meadows for primary habitat, nursery habitat, or feeding grounds, and herbivores utilize these seagrass meadows as both a habitat and food source. Seagrass is the primary food source for adult green sea turtles (Chelonia mydas) and the Florida manatee (Trichechus manatus latirostris). In the northern Atlantic Ocean, 297 fish species have been documented to use seagrasses, and this number is higher in other parts of the world.
Seagrasses protect coastal communities from flooding, storm surges, and erosion. Seagrass canopies dissipate wave energy and trap sediments, while seagrass roots and rhizomes secure sediments in place. Unlike traditional “gray” infrastructure (e.g., seawalls, bulkheads), seagrasses can adapt to rising sea levels, creating a lasting tool that reduces the negative impacts of sea level rise and extreme weather events, while also providing myriad additional benefits.
Lastly, seagrass meadows can boost local economies by providing tourism opportunities and supporting recreational and commercial fisheries. Because seagrasses provide habitat to many organisms including scallops, sea turtles, manatees, rays, crabs, and many types of fish, seagrass ecosystems are used for recreational activities, such as snorkeling and fishing. Fisheries in Florida rely heavily on seagrasses, especially those that target scallops or game fish like grouper and snapper. In the Indian River Lagoon in Florida, seagrasses have a fishery-based value of $5,000–$10,000 per acre per year (Hazen and Sawyer 2008; SJRWMD 2012). Seagrass meadows provide an array of benefits to Florida’s ecosystems and residents, which necessitates protection and conservation of these seagrass meadows.
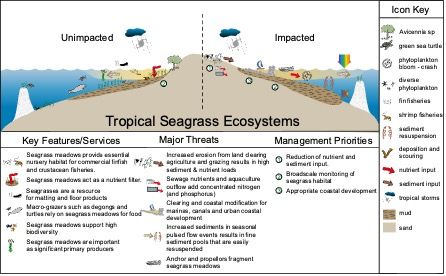
Threats to Seagrass Ecosystems
Seagrasses are rapidly declining worldwide, with nearly one-third of the global areal seagrass cover lost between 1879 and 2006. An additional 7% of seagrass is estimated to be lost every year (Waycott et al. 2009). With this loss of seagrass cover comes loss of the many benefits that seagrasses provide to humans and the environment. For example, stored carbon is released into the atmosphere following seagrass die-offs, which can accelerate the rate of climate change (Fourqurean et al. 2012).
Coastal development and declining water quality are major drivers of seagrass loss. When sediment and nutrient runoff enters seagrass meadows, suspended sediments and algal blooms can shade the seagrass, leading to seagrass loss. Additionally, physical disturbances (e.g., dredging, boat propeller scars, construction of docks and marinas, and damage from canal estates and industry) directly remove seagrasses.
Climate change can also negatively impact seagrasses. Storms and extreme weather events are becoming more frequent, and they bring physical damage to seagrasses and reduce water quality. For example, droughts can result in high salinity events (outside of seagrass physiological limits), and storms can change water quality by carrying excess nutrients and pollutants into coastal areas. Additionally, warming temperatures and heat waves can negatively impact seagrasses. As temperatures increase, respiration increases at a faster rate than photosynthesis, which can result in carbon limitation for plants (Marsh et al. 1986). High respiration rates (due to warmer temperatures or decomposition) also reduce water column and sediment oxygen levels, which can be harmful to seagrasses.
Warming temperatures indirectly impact seagrasses through tropicalization (i.e., the range expansion of tropical species into subtropical regions) (Heck et al. 2015). In the northern Gulf of Mexico, populations of tropical herbivores including green turtles, manatees, and emerald parrotfish are increasing due to tropicalization and conservation efforts. This is expected to yield increased grazing pressure on seagrasses, potentially reducing seagrass structure and density.
These threats to seagrasses are not occurring in isolation. Multiple stressors are concurrently impacting seagrass meadows. When multiple stressors co-occur, this can create unexpected, non-additive interactions that can potentially result in a more severe impact to seagrasses.
What is seagrass diversity, and why does it matter?
Seagrass diversity has two main components: species diversity (i.e., the number of seagrass species and their relative abundance) and genetic diversity (i.e., the number of genetically distinct individuals within a single species and their relative abundance). Because seagrasses reproduce both asexually (producing a genetically identical clone of the parent) and sexually (producing a genetically unique plant), seagrasses have served as a model organism for studying genetic diversity. While many temperate regions only contain one seagrass species, subtropical and tropical regions can contain multiple co-occurring seagrass species. There are seven seagrass species found in Florida: turtle grass (Thalassia testudinum), manatee grass (Syringodium filiforme), shoal grass (Halodule wrightii), star grass (Halophila engelmannii), paddle grass (Halophila decipiens), Johnson’s seagrass (Halophila johnsonii), and widgeon grass (Ruppia maritima). Genetic diversity of Thalassia, Syringodium, and Halodule has been found to differ between populations and locations.
Researchers have observed many positive effects of seagrass genetic diversity on ecosystem functions and stability. Seagrass genetic diversity can increase resistance to disturbances (i.e., ability of the system to remain unchanged following a disturbance) and resilience to disturbances (i.e., the ability of the system to recover following a disturbance). Genetic diversity in seagrasses can increase ecosystem functions as well. Examples include augmenting invertebrate habitat, nutrient retention, and primary productivity, and increasing the value of seagrasses.
There are multiple mechanisms that can produce positive effects of diversity on ecosystem functions and stability. Seagrass genotypes and species have different traits, which can result in facilitation, sharing of resources, and different responses to disturbances (Hughes and Stachowicz 2011; Reynolds et al. 2016). For example, taller plants can protect sensitive understory plants from excess light, thereby facilitating the survival of shorter plants (Dawson and Dennison 1996). Additionally, seagrass genotypes and species have different rooting depths (Williams 1990), allowing them to draw from different nutrient pools and reduce competition for resources. Finally, response to disturbances varies based on the species or genotype, allowing more resistant species to compensate for losses in more sensitive species and increasing ecosystem stability (Yachi and Loreau 1999). While researchers have focused on the positive impacts of seagrass genetic diversity, we expect the same mechanisms to produce positive impacts of seagrass species diversity.
Incorporating Diversity into Seagrass Management and Restoration
Seagrass diversity (both species and genetic) is an important factor to consider in management and restoration decisions. Restoration projects that maximize seagrass genetic and species diversity have been shown to have increased success, measured both as plant persistence and as the enhanced provision of ecosystem functions (Reynolds et al. 2012). Incorporating seagrass species richness into restoration trials can also enhance restoration success, with higher plant survival and growth rates (Williams et al. 2017). Similarly, genetic diversity can improve restoration success via higher seagrass survival, shoot density, and primary productivity, which increases nutrient retention and invertebrate density (Reynolds et al. 2012). Because genetic diversity increases resistance and resilience to environmental stressors (including grazing, heat waves, and low light), restored seagrass meadows that contain high genetic diversity will be better able to withstand and recover from environmental stress (Hughes and Stachowicz 2004; Reynolds et al. 2012; Reynolds et al. 2019).
Monitoring seagrass diversity can provide managers and researchers with insight into ecosystem stability. Losses in seagrass diversity may indicate increased vulnerability to disturbances and the need for intervention. A large-scale seagrass die-off in the Indian River Lagoon in Florida resulted in higher seagrass population differentiation, indicating genetic drift and dispersal limitation, which may impede large-scale recovery. In short, these disturbances resulted in genetic changes that may have impacts on seagrass ecosystem functions. Therefore, researchers are calling for preservation of existing genetic diversity in the Indian River Lagoon via captive breeding programs (i.e., seagrass nurseries) (Reynolds et al. 2019). Identifying dispersal limitations suggests the importance of active restoration to speed the recovery of seagrass populations (Reynolds et al. 2013).
Information on the genetics of natural seagrass populations as well as seagrass nurseries is critical for selecting donor material for restoration projects. Donor material should be locally adapted (i.e., match genetics of local populations) and contain high genetic diversity. Monitoring the diversity of restored meadows can provide insight into the success of the restoration project. Restored seagrasses should maintain similar diversity levels as the donor population. With high rates of seagrass loss and increasing anthropogenic pressure, creating restored seagrass meadows that are resistant and resilient to environmental disturbances is crucial. Maximizing seagrass genetic diversity can provide this enhanced stability. Restoring stable seagrass meadows can ultimately lead to the recovery of many valuable services that benefit ecosystems and humans.
References
Dawson, S. P., and W. C. Dennison. 1996. “Effects of Ultraviolet and Photosynthetically Active Radiation on Five Seagrass Species.” Marine Biology 125:629–638. https://doi.org/10.1007/BF00349244
Flindt, M. R., M. A. Pardal, A. I. Lillebo, I. Martins, and J. C. Marques. 1999. “Nutrient Cycling and Plant Dynamics in Estuaries: A Brief Review.” Acta Oecologica 20 (4): 237–248. https://doi.org/10.1016/S1146-609X(99)00142-3
Fourqurean, J. W., C. M. Duarte, H. Kennedy, N. Marba, N. Holmer, M. A. Mateo, E. T. Apostolaki, G. A. Kendrick, D. Krause-Jensen, K. J. McGlathery, and O. Serrano. 2012. “Seagrass Ecosystems as a Globally Significant Carbon Stock.” Nature Geoscience 5:505–509. https://doi.org/10.1038/NGEO1477
Hazen and Sawyer Environmental Engineers and Scientists. 2008. “Indian River Lagoon Economic Assessment and Analysis Update.” Final Report to IRL National Estuary Program (Contract 24706), Palm Bay, Florida.
Heck, K. L. Jr., F. J. Fodrie, S. Madsen, C. J. Maillie, and D. A. Byron. 2015. “Seagrass Consumption by Native and a Tropically Associated Fish Species: Potential Impacts of the Tropicalization of the Northern Gulf of Mexico.” Marine Ecology Progress Series 520:165–173. https://doi.org/10.3354/meps11104
Hughes, A. R., and J. J. Stachowicz. 2004. “Genetic diversity enhances the resistance of a seagrass ecosystem to disturbance.” Proceedings of the National Academy of Sciences 101 (24): 8998–9002. https://doi.org/10.1073/pnas.0402642101
Hughes, A. R., and J. J. Stachowicz. 2011. “Seagrass genotypic diversity increases disturbance response via complementarity and dominance.” Journal of Ecology 99 (2): 445–453. https://doi.org/10.1111/j.1365-2745.2010.01767.x
Marsh Jr., J. A., W. C. Dennison, and R. S. Alberte. 1986. “Effects of Temperature on Photosynthesis and Respiration in Eelgrass (Zostera marina L.).” Journal of Experimental Marine Biology and Ecology 101 (3): 257–267. https://doi.org/10.1016/0022-0981(86)90267-4
Moncreiff, C. A., M. J. Sullivan, and A. E. Daehnick. 1992. “Primary Production Dynamics in Seagrass Beds of Mississippi Sound: The Contributions of Seagrass, Epiphytic Algae, Sand Microflora, and Phytoplankton.” Marine Ecology Progress Series 87:161–171.
Reynolds, L. K., K. J. McGlathery, and M. Waycott. 2012. “Genetic diversity enhances restoration success by augmenting ecosystem services.” PLOS ONE 7 (6): e38397. https://doi.org/10.1371/journal.pone.0038397
Reynolds, L. K., M. Waycott, and K. J. McGlathery. 2013. “Restoration recovers population structure and landscape genetic connectivity in a dispersal‐limited ecosystem.” Journal of Ecology 101 (5): 1288–1297. https://doi.org/10.1111/1365-2745.12116
Reynolds, L. K., K. DuBois, J. M. Abbott, S. L. Williams, and J. J. Stachowicz. 2016. “Response of a habitat-forming marine plant to a simulated warming event is delayed, genotype specific, and varies with phenology.” PLOS ONE 11 (6): e0154532. https://doi.org/10.1371/journal.pone.0154532
Reynolds, L. K., K. A. Tiling, G. B. Digiantonio, V. G. Encomio, and L. J. Morris. 2019. “Genetic diversity of Halodule wrightii is resistant to large scale dieback: A case study from the Indian River Lagoon.” Conservation Genetics 20:1239–1337. https://doi.org/10.1007/s10592-019-01214-z
Waycott, M., C. M. Duarte, T. J. B. Carruthers, and S. L. Williams. 2009. “Accelerating loss of seagrasses across the globe threatens coastal ecosystems.” Proceedings of the National Academy of Sciences 106 (30): 12377–12381. https://doi.org/10.1073/pnas.0905620106
Williams, S. L. 1990. “Experimental Studies of Caribbean Seagrass Bed Development.” Ecological Monographs 60 (4): 449–469.
Williams, S. L., R. Ambo-Rappe, C. Sur, J. M. Abbott, and S. R. Limbong. 2017. “Species richness accelerates marine ecosystem restoration in the Coral Triangle.” Proceedings of the National Academy of Sciences 114 (45): 11986–11991. https://doi.org/10.1073/pnas.1707962114
Yachi, S., and M. Loreau. 1999. “Biodiversity and Ecosystem Productivity in a Fluctuating Environment: The Insurance Hypothesis.” Proceedings of the National Academy of Sciences 96 (4): 1463–1468. https://doi.org/10.1073/pnas.96.4.1463