Introduction
The excitement of watching a seed sprout into a flourishing plant is a joy for all who cultivate their own urban gardens. Nevertheless, many think of soil as a mere support for those plants, a substance that anchors them into the ground. However, soil is far more than just dirt; it is a dynamic and life-sustaining medium that nourishes plants. In fact, there is a complex ecosystem beneath the soil surface, composed of minerals, organic matter, water, air, and countless microorganisms. Therefore, it is crucial to understand some soil relations to comprehend the nutrient needs of plants and provide them with the proper nutritional care. Whether a person is growing flowers on a balcony or cultivating vegetables in raised beds, understanding soil fertility is vital. Soil fertility refers to the ability of soil to sustain plant growth (Hodges 2010). Fertile soil provides the necessary nutrients, structure, and microbial activity crucial for plants to thrive (Figure 1).
This publication is written for individuals not actively engaged in traditional agriculture but rather those tending to lawns, shrubs, trees, or flowers in urban settings. This publication will not only introduce readers to some essential terms of soil fertility but also explain the role of soil properties in supporting healthy plants. Furthermore, it familiarizes readers with methods to enhance soil fertility, offering practical strategies for optimal plant growth within urban settings. It serves as a guide to empower urban residents, offering insights into plant nutrient requirements and the principles of effective nutritional care.

Credit: alisonhancock - stock.adobe.com
Commonly Used Soil Fertility Terminology
Plant Nutrients
A plant's life cycle, which includes optimal growth and reproduction, necessitates the presence of 17 different nutritional elements. These elements are essential to a plant's survival. Carbon (C), hydrogen (H), and oxygen (O) are all supplied by air and water, with limited supply management capabilities. These three elements, constituting at least 94% of a plant's dry tissue, form the foundation of plant structure and function. However, deficiencies in the remaining 14 elements can inhibit plant growth, with nitrogen (N) deficiency being the most common issue. These essential elements, both macronutrients and micronutrients, are crucial for a plant's development. Macronutrients such as N, phosphorus (P), potassium (K), sulfur (S), calcium (Ca), and magnesium (Mg) are required in larger quantities, while micronutrients, including manganese (Mn), iron (Fe), boron (B), zinc (Zn), copper (Cu), molybdenum (Mo), chlorine (Cl), and nickel (Ni), are needed in smaller amounts. Whether sourced from the soil or supplemented through fertilizers, these nutrients play pivotal roles in plant growth and development. The soil's mechanisms for absorbing, fixing, and releasing these nutrients can significantly impact their availability to plant roots, directly influencing plant vitality and growth. Understanding these nutrient categories and their soil interactions is essential for cultivating healthy and thriving plants (Mengel et al. 2001).
Table 1. Essential plant nutrients and their function in plants.
Soil Organic Matter
Organic matter (OM) in the soil encompasses various components such as plant residues, microbes, detritus, and additional microorganisms. The residual material from once-living organisms is referred to as OM. As these plant materials undergo decomposition by microbes, they eventually transform into humus, which represents a more stable form of organic matter. This humus, and OM, plays a pivotal role in soil health and structure. When incorporated into sandy soil, OM improves water retention. OM is also added to clay or silt soils to improve the soil structure by helping the soil particles stick together better. OM creates tiny spaces in the soil, allowing the plant roots to get air that is essential for their growth. The soil will drain well and be easier to handle. OM also encourages the growth of microorganisms in the soil that keep it healthy (Baldock and Nelson 2000). OM provides nutrients as it decomposes, buffers the pH of the soil solution against rapid chemical changes, and improves soils’ cation exchange capacity (CEC).
Soil pH
Soil pH is a measure of soil's acidity or alkalinity level. The pH scale ranges from 0 (very acidic) to 14 (very alkaline). A soil pH reading below 7.0 indicates that the soil is acidic, whereas readings above 7.0 suggest an alkaline condition. Soils generally range from pH 4.0 to pH 8.0. Soil pH is important because the availability of nutrients to your plants is closely linked to the acidity or alkalinity of your soil. When your soil has a pH between 6.0 and 7.0, it is in perfect condition for plants to access important nutrients. If your soil is too acidic, the presence of nutrients like K, Ca, and Mg is limited, while potentially harmful elements like Al, Fe, and Zn tend to accumulate. Conversely, alkaline soils often exhibit deficiencies in Fe, Mn, Zn, and B (Figure 2). So, maintaining the right pH balance is key to ensuring your plants get the nutrients they need to thrive (Neina 2019).
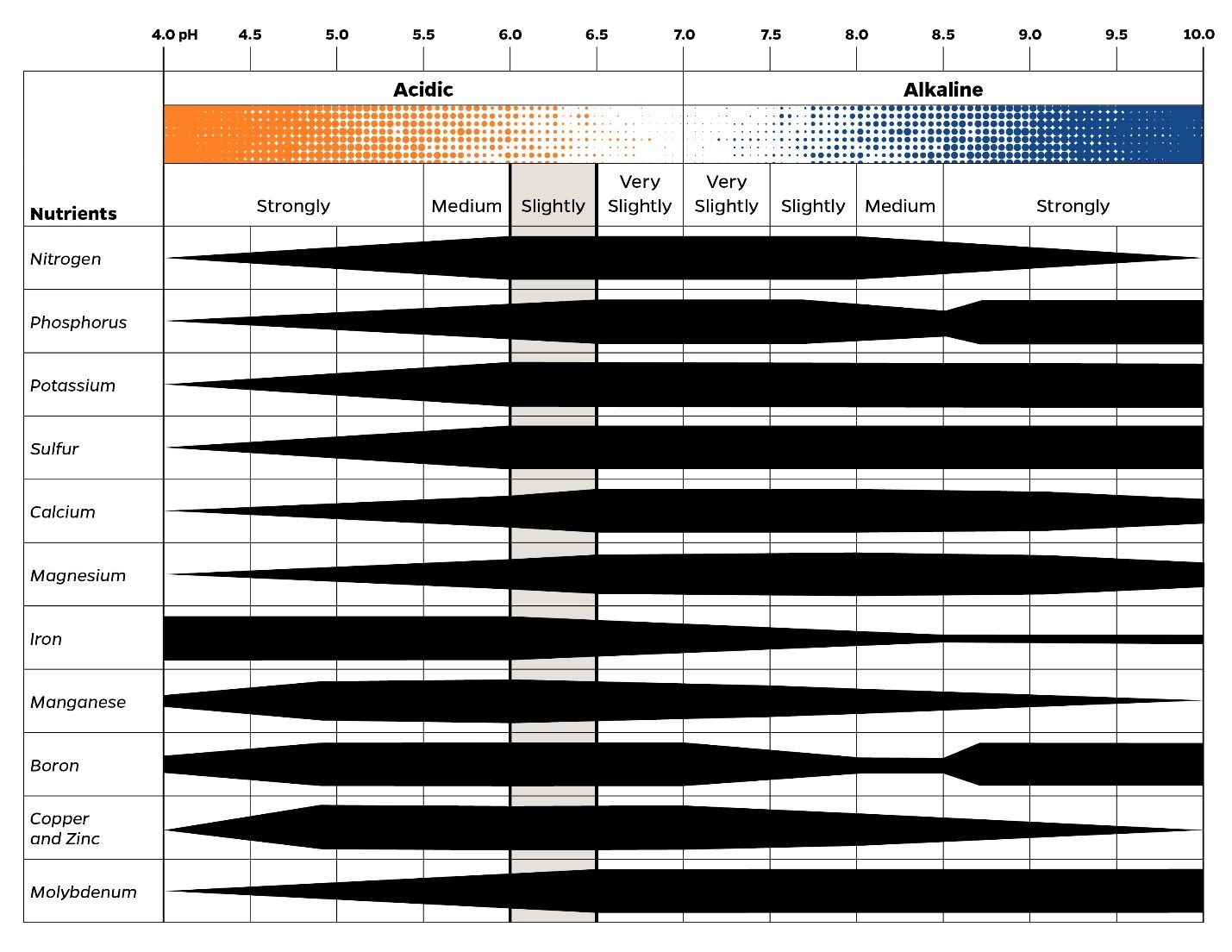
Credit: UF/IFAS
Cation Exchange Capacity
Cation exchange capacity (CEC) in soil refers to the soil's ability to retain and exchange positively charged ions or cations, such as calcium (Ca2+), magnesium (Mg2+), potassium (K+), and hydrogen (H+). Basically, CEC can be likened to the soil's storage and exchange system for essential nutrients. Think of it as the soil's capacity to hold onto and release nutrients to plant roots. Soils with a higher CEC generally have greater nutrient-holding capacity, providing a more stable reservoir of essential elements for plant growth. Cation exchange capacity is measured in units of milliequivalents of charge per 100 g of dry soil (meq/100 g). The higher the CEC value, the greater the soil's ability to retain and supply cations to plants (Figure 3), which can positively impact soil fertility overall (Chapman 1965).
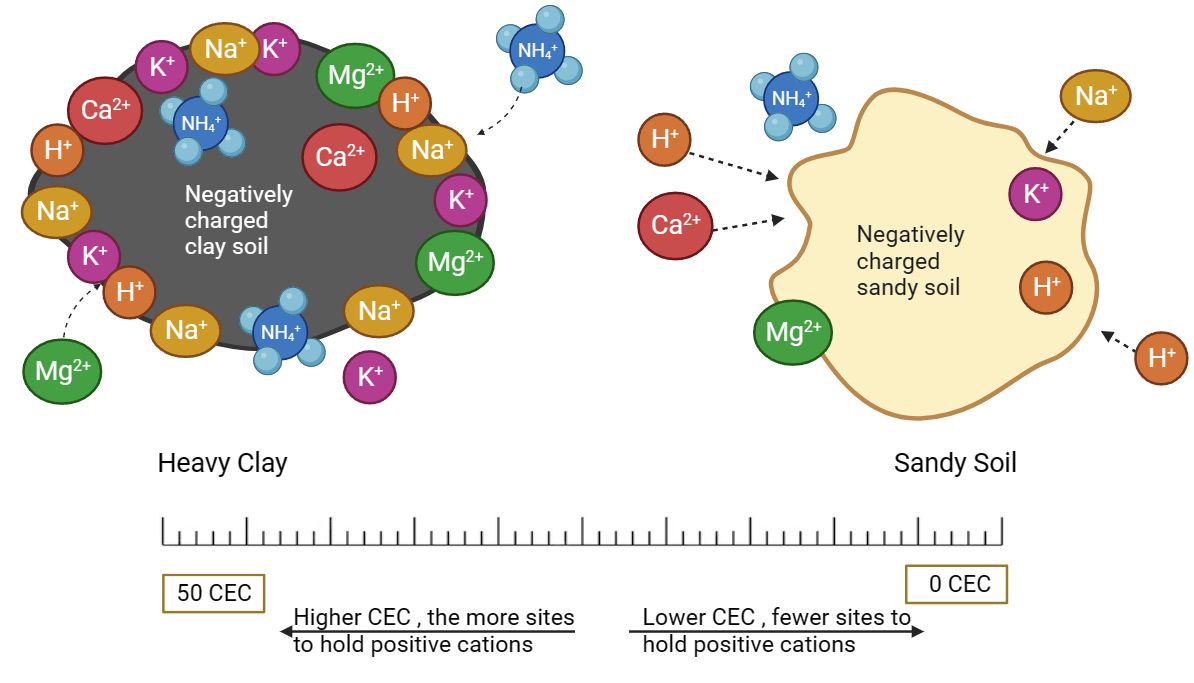
Credit: Ravinder Singh, UF/IFAS
Improving Soil Fertility
Soil Testing
The first step in improving soil fertility is to examine the soil and determine nutrient availability and deficiencies. A balance of available nutrients, pH, CEC, and OM percentage is essential for optimal plant growth. The only way to know is to conduct a soil fertility test. This analysis provides crucial insights into the nutrient composition, pH levels, OM percentage, CEC, and overall health of your soil. With this information, you can tailor your fertilization practices to meet the specific needs of your plants, avoiding overuse and potential environmental impacts. Fertilizing without the soil test results can lead to a waste of money and product and can exacerbate an existing nutrient imbalance. Maintaining a proper balance of available nutrients and keeping other soil properties in their normal ranges (Soil pH normal range: 6.0–7.0) are vital in achieving the best possible conditions for plant growth. You have two choices for testing your soil sample. The traditional approach is to send it to the UF/IFAS Extension Soil Testing Laboratory (Figure 4). Alternatively, you can opt for the newer SoilKit® testing kit, which is the result of a partnership between UF/IFAS and AgriTech Corp (Figure 5). This kit offers a user-friendly soil testing analysis with easily understandable solutions for consumers. Both testing options are conveniently available at your local county Extension office and are based on UF/IFAS research. For your specific requirements, county Extension agents can help you choose the most suitable test. See EDIS publication #SL281, “Soil Sampling and Testing for the Home Landscape or Vegetable Garden” (https://edis.ifas.ufl.edu/publication/SS494), for information about how to take soil samples properly.

Credit: Marisol Amador, UF/IFAS

Credit: Tyler Jones, UF/IFAS
Determining If the Plants Need Nutrients from External Sources
Soil test results offer a crucial insight into your soil's nutritional status, identifying any deficiencies that may impede healthy plant growth. Addressing these deficiencies is vital to ensure the soil adequately supports plant life. Typically, plants require the application of supplemental nutrients to complete their life cycle. However, the application of fertilizer depends on the plant needs and desired results, for example, gardening or aesthetic appeal. For instance, a farmer who intends to boost crop yield applies fertilizer when growing plants. Similarly, homeowners and residents may apply fertilizer for nutritional needs such as lawns, trees, shrubs, flowers, nurseries, or kitchen gardens. Since these urban gardens may not aim for high commercial yields, homeowners might decide to grow without using fertilizers. Yet, if signs of nutrient deficiency emerge, such individuals might consider supplementing nutrients. Figures 6 and 7 comprise nutrient deficiency symptoms that may help determine which nutrient to supplement.
The most frequent nutrient deficiency in urban contexts is N due to transported soils (required for house strength in Florida) or due to the leaching ability of N. Typical N shortage symptoms manifest on the older leaves of any plant due to the mobile nature of N inside the plant. The second most essential nutrient is P, which is regarded to be abundant in Florida's soils; consequently, it is recommended not to add P fertilizer until the soils are low. The use of excess P fertilizer may contribute to water contamination, such as eutrophication of water bodies. Due to the mobile nature of K in the soil profile, especially in Florida’s sandy soils with very low CEC, K is highly prone to leaching. Therefore, K fertilizer could be administered in urban conditions.
Micronutrients like B, Mn, and Mo are essential in tiny quantities but are not typically included in standard fertilizer blends. Many gardeners don't actively track or consider these nutrients unless their plants exhibit issues, such as the development of hollow heart in broccoli, which can result from a B deficiency. Some of these micronutrients can be obtained from local farm supply stores. Another way to enhance micronutrient levels in the soil is by using liquid fertilizers that contain these elements or by applying various types of quality compost or animal manure.
If your garden has sandy soils and low CEC, a beneficial approach involves frequent but smaller applications of fertilizer. Conversely, in a garden with a high level of clay and higher CEC, adding higher fertilizer rates is recommended less frequently.
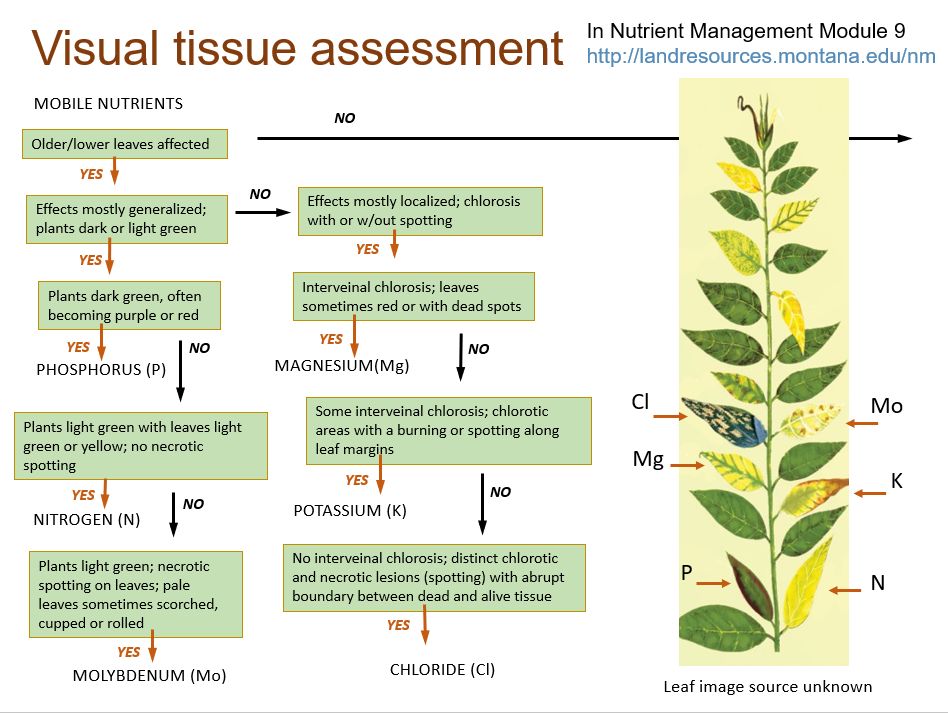
Credit: Montana State University (https://landresources.montana.edu/soilfertility/nutrientdeficiency.html)
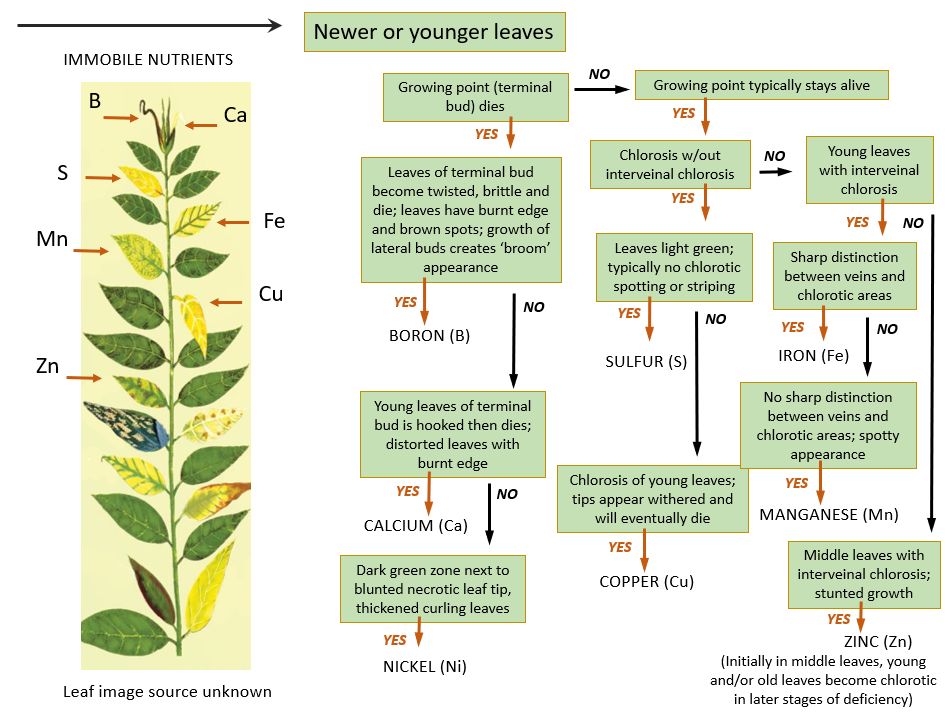
Credit: Montana State University (https://landresources.montana.edu/soilfertility/nutrientdeficiency.html)
Maintaining pH of Soil
Balancing the soil's pH is essential for ensuring plants receive the necessary nutrients for optimal growth. Depending on the soil test outcomes, amendments may be necessary to adjust the soil pH to an optimal range. If the soil is too acidic, applying lime can raise the pH, whereas an overly alkaline soil may require elemental sulfur to lower the pH. The soil test report provides specific recommendations on the quantity of lime or sulfur needed for effective correction. Do not put lime or gypsum in soil without an accurate soil test. Adjusting soil pH without a soil test is dangerous as it can kill the plants completely. For example, if your soil is slightly acidic and you add elemental sulfur without knowing the soil pH, it could make the soil even more acidic. This strong acidity might lead to a lack of nutrients in the soil and can kill the plants (Figure 2). For more details on how to manage soil pH, see EDIS publication #SL256, “Soil pH and the Home Landscape or Garden” (https://edis.ifas.ufl.edu/publication/SS480).
Increasing Organic Matter
Adequate OM is one of the most important indicators of soil health and structure. To ensure healthy plant growth, it's essential to create soil conditions that facilitate adequate air space, ample room for root development, and the right balance of soil pH, water, sunlight, and essential nutrients. The availability of air space, root room, and water for plants is primarily determined by the soil's structure. This, in turn, depends on the amount of OM in the soil and the proper distribution of mineral particles in various sizes. Sources of OM for soil enrichment encompass items like compost, animal manure, crop residues, and cover crops. In your own yard, these sources may also comprise of fallen leaves, grass clippings, and kitchen compost. We will discuss compost and other organic amendments further in this publication.
Protecting the Soil with Organic Mulch
The application of organic mulch over garden soil offers numerous advantages when executed correctly. Acting as a protective layer, organic mulches retain soil moisture, moderate temperature fluctuations, suppress weed growth, enrich soil structure through decomposition, and aid in rainwater retention. Materials like shredded bark, hardwood, leaves, compost, pine needles, bark nuggets, wood chips, and straw serve as effective organic mulches with uniform and adequate depth, ensuring optimal soil protection and enhancement. By employing organic mulches, gardeners maintain healthier soil conditions, fostering an environment conducive to thriving plants. For further details on Florida friendly mulches, see EDIS publication #ENH1362, “Florida-Friendly Mulches and Their Uses” (https://edis.ifas.ufl.edu/publication/EP626).
Using Compost or Other Organic Amendments to Improve Soil Fertility
As we discussed in a previous section of this publication, adequate OM is one of the most important indicators of soil health. The availability of air space, root room, and water for plants is primarily determined by the soil's structure. Therefore, creating the right soil conditions depends on the amount of OM in the soil and the proper distribution of mineral particles in various sizes. However, urban landscapes have often gone through a number of residential, commercial, or industrial uses in the past, and this land-use history presents unique challenges to aspiring urban farmers or gardeners. Urban soils intended for food production often start in poor shape: they are usually compacted and have low OM content, low nutrient availability, and low biological activity and diversity. When the soil is compacted or has low OM, the best option is probably to add and mix organic fertilizers.
In their natural, moist state, organic fertilizers encompass all types of animal manure and compost created from a mixture of manure and other plant or animal-derived materials. Commercial organic fertilizers, on the other hand, include products like dried manures, bone, and blood meal, as well as cottonseed and soybean meals.
Credit: Ravinder Singh, UF/IFAS
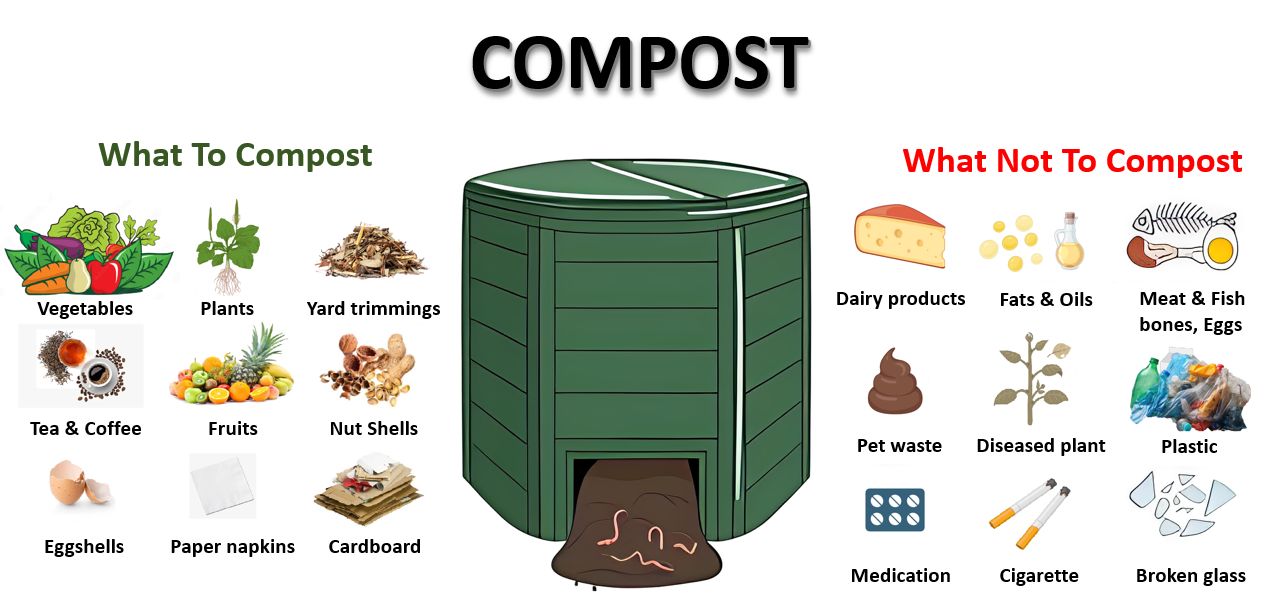
Compost serves as a valuable organic matter source for soil. Moreover, composting plays a pivotal role in completing the recycling cycle by converting waste materials into beneficial soil amendments. Compost can be produced at home or bought from commercial sources. To produce your own compost, you'll need to construct or acquire a compost bin. Compost is created by stacking organic materials and allowing them to decompose over time, resulting in a nutrient-rich, humus-like substance. Compost generally contains modest quantities of N and K, with relatively low levels of P, but it does typically contain essential micronutrients. Homemade compost serves as an excellent source of nutrients and organic matter for garden soils. It can be created from a variety of materials, including grass clippings, tree leaves, and kitchen waste like salad trimmings and vegetable scraps. Additionally, sawdust from untreated wood, pine needles, and animal manure from herbivores such as chickens, cows, horses, goats, and lambs can also be used in composting. Never use cat or dog manure or human waste, as there is a higher risk of these sources transmitting disease (Figure 5). For more information on composting, refer to EDIS publication #ENH1065, “Compost Tips for the Home Gardener” (https://edis.ifas.ufl.edu/publication/EP323).
Worm composting, also known as vermicomposting, is also a unique method of composting that is particularly efficient for repurposing food waste. You can either build your own worm bins or purchase them in various designs, and obtaining the required red wiggler worms is quite straightforward. These worms are fed with a diet of vegetable and fruit scraps, coffee grounds, pasta, bread, cereal, tea bags, newspaper, and other paper products. They are highly efficient eaters and will convert these materials into valuable compost known as castings, as well as "worm tea," a nutrient-rich liquid that plants thrive on. When properly maintained, many worm bins can be kept indoors or on a porch without causing any unpleasant odors. It's important to create and maintain a habitat that is dark, cool, and adequately moist (but not overly wet). Ensure the bin has adequate air holes and keep it out of direct sunlight, as outdoor worm bins can become too hot and harm the worms if not carefully managed.
For pre-planting application, use approximately 25 to 100 pounds of manure per 100 square feet of garden. After planting, you can side-dress with up to 5 pounds per 100 square feet of row. If your garden is mulched, first move the mulch away from the plants, apply the manure, and then replace the mulch once you've incorporated the manure into the soil.
Where to Buy Chemical Fertilizer and How to Determine the Required Amount
Since OM does not have a balanced mix of essential nutrients, using a commercial fertilizer could be necessary to ensure that plants receive all the required nutrients. Supplying nutrients for healthy plant growth is crucial, but excessive amounts can lead to environmental pollution. The excessive release of nutrients from over-irrigation or excessive rainfall can result in harmful consequences, especially when they enter water bodies. This nutrient runoff can trigger excessive algae growth (i.e., eutrophication), posing threats to the well-being of aquatic life, including fish and other creatures. Therefore, understanding the appropriate nutrient application becomes imperative. Fertilizing should be carried out only when plants require nutrients, following UF/IFAS recommendations for precise rates and timings.
Soil fertility management for urban audiences falls under the umbrella of home lawn management. Detailed information on fertilization requirements for Florida lawns is available in the EDIS publication #ENH979, “Best Management Practices for the Home Lawn” (https://edis.ifas.ufl.edu/publication/EP236). To address environmental concerns, the urban turf fertilizer rule has been established, aiming to minimize N and P losses and water pollution caused by improper fertilizer application. This regulation will necessitate adherence to specific guidelines for N and P application rates by fertilizer applicators, including homeowners and landscape professionals. Further insights into this rule are provided in the EDIS publication #ENH1089, “Urban Turf Fertilizer Rule for Home Lawn Fertilization” (https://edis.ifas.ufl.edu/EP353).
There are several resources available for the non-farmer to purchase the required fertilizer. Common places from where most landowners buy their fertilizers are Lowe's, Home Depot, Tractor Supply, and so forth. Price, availability, ease of use, necessary equipment, time, and philosophy should be considered when selecting the best fertilizer and application method for any situation. Occasionally, in severe nutrient deficiency situations, some micronutrients, such as magnesium and iron, are sprayed onto the foliage of crops, but most are applied to the soil and taken up by roots. The numbers, often referred to as the analysis, displayed on the label or the front of a fertilizer bag indicate the proportions of N, P, and K contained in the fertilizer. The most common fertilizers available in these shops are 10-10-10 or 6-6-6; this means the N, P, and K in the bags are each in the ratio of 10% or 6%, respectively. Manufacturers process these chemicals into forms that are well-suited for plant utilization. Note that the plant nutrients found in fertilizers are identical to those naturally acquired from the soil. These natural nutrients in soil become available to plants through the activities of soil organisms on OM.
Slow-release fertilizers are also available in these shops but are relatively more expensive than conventional fertilizers. Consult the UF/IFAS Extension Soil Testing Lab for soil analysis to determine the nutrient status of your soil. After soil testing, you can interpret the required fertilizer application according to the following calculation examples.
Example 1
Question: Assume a fertilizer bag weighs 50 pounds with 10-10-10 N, P, and K. How much of each nutrient is available?
Answer: The active ingredient of each nutrient is 10% in 50 pounds. Therefore, 10% of 50 pounds is 5 pounds.
Example 2
Question: Suppose I want to fertilize a 1,000 sq ft area with a 50 pounds per acre rate of N. How much N do I need?
Answer:
1 acre = 43560 sq ft
For 43560 sq ft, we need 50 pounds
For 1 sq ft = 50/43560
For 1000 sq ft = (50/43560) × 1000 = 1.15 pounds of N is needed to fertilize 1000 sq ft at 50 pounds/acre.
Example 3
Sometimes, N is available in urea bags at the local vendor shop. The percent N in urea is 46; therefore, it is important to calculate how much urea is needed to apply a specific amount of N, which in the previous example is 1.15 pounds.
Question: How much urea is needed to apply 1.15 pounds of N?
Answer:
Percent N in urea = 46
Required N = 1.15 pounds
Let’s calculate the amount of urea required for 1 pound. It will make any calculation easy.
For 46 pounds of N, 100 pounds of urea is needed.
For 1 pound of N = 100/46
For 1.15 pounds of N = 100/46 × 1.15 = 2.50 pounds of urea is needed to apply 1.15 pounds of nitrogen.
Similar calculations could be done for other nutrients.
Take Home Messages
The cornerstone of achieving a flourishing urban garden lies in the comprehensive understanding and meticulous management of soil fertility.
The soil composition forms the very foundation upon which successful urban gardening is built. By adopting organic practices, one not only nurtures the soil but also contributes to the sustainability and environmental health of the urban ecosystem.
Regular testing and amending of the soil, based on its specific needs, are crucial steps that ensure the soil retains its vitality and fertility.
These practices, when diligently applied, set the stage for a garden that is not only vibrant in its appearance but also thriving in its productivity, thereby contributing significantly to the urban landscape and the well-being of its inhabitants.
References
Baldock, J. A., and P. N. Nelson. 2000. “Soil Organic Matter.” In Handbook of Soil Science, edited by M. E. Sumner. CRC Press.
Chapman, H. D. 1965. “Cation‐Exchange Capacity.” In Methods of Soil Analysis: Part 2 Chemical and Microbiological Properties, edited by A. G. Norman. https://doi.org/10.2134/agronmonogr9.2.c6
Hodges, S. C. 2010. Soil Fertility Basics: NC Certified Crop Advisor. North Carolina State University Soil Science Extension. https://www.online-pdh.com/file.php/562/FER_SG.pdf
Mengel, K., E. A. Kirkby, H. Kosegarten, and T. Appel. 2001. “Plant Nutrients.” In Principles of Plant Nutrition. Springer Dordrecht https://doi.org/10.1007/978-94-010-1009-2_1
Neina, D. 2019. “The Role of Soil pH in Plant Nutrition and Soil Remediation.” Applied and Environmental Soil Science 2019: 1–9. https://doi.org/10.1155/2019/5794869