Introduction
Exotic, nonnative, or alien species are animals or plants that, as a result of human activity or interaction, are living outside their native range. Only a small portion of these species is considered invasive, a word that refers to an organism's potential to harm an ecosystem, human health, or the economic stability of an area. Captures and postmortem examinations, or necropsies, of invasive or nonnative animals may provide insight into the ecological impact of these invaders.
Our goal is to build capacity for researchers to perform necropsies when an opportunity arises to examine nonnative species for study. While the techniques described serve as a guide to necropsy reptiles, we focus on exotic species found in south Florida. South Florida provides prime habitat for invasive reptiles such as Argentine black and white tegu (Tupinambis merianae) and Burmese python (Python molurus bivittatus), and these species are used to describe our techniques.

Credit: Robin Bijlani
Importance
Understanding basic ecology and biology of invasive species is integral to their management. Necropsies are a tool to discover how an invasive species interacts with the ecosystem. Necropsies provide essential data about a species' disruptive potential (through diet analysis), reproductive potential (via sperm and egg counts), and overall health (disease, parasites, and body condition). In a lab, we further our understanding of a species' impact through histology, bacteriology, biochemistry and genetics. This information provides valuable insight applicable to management practices and informs efforts to reduce populations of invasive species.
Protocols
Intake Data
A complete history of the study specimen should be included with necropsy findings, including capture information (e.g., personnel and agency, date, time, location with spatial coordinates), age class (hatchling, juvenile, sub-adult or adult), sex, weight and length measurements, and any other information regarding the animal's life history (including diet while in captivity, etc.). If the animal is already dead, it is important to document how it died (e.g., physical or chemical euthanasia, struck by vehicle, etc.) and when.
Animal Handling
Live animals should be secured in cloth bags made of breathable material such as cotton (even a pillowcase or mesh bag will suffice) and transported back to the lab in a secure, ventilated enclosure such as a Rubbermaid ActionPacker® with small holes drilled into it, or a pet crate. Take great care with venomous snakes to protect yourself.
Sample Collection
Consider what tissue or blood samples may be needed for analysis before you capture and euthanize an animal. Design a sampling protocol to ensure that valuable samples and information are collected in a systematic fashion. The sample type, collection procedure, packaging, and storage may vary depending on the toxicant or compound being analyzed (Jacobson 2007). For example, it may be necessary to collect samples aseptically by either sterilizing your instruments between different organ collections or by using different sterile tools. Table 1 briefly discusses some of the commonly collected samples.
Blood
Evaluation of complete blood counts (CBCs) include red blood cell (RBC) count, hemoglobin concentration (Hb), packed cell volume (PCV), white blood cell (WBC) count, differential leukocyte counts, and morphologic evaluation of blood cells. Biochemical evaluations of blood generally involve analysis of plasma or serum samples (Jacobson 2007). Blood samples to be used for CBC or plasma chemistry analysis should be immediately placed in an anticoagulant, while samples for serum chemistry analysis should be collected into a clot tube containing a serum separator gelatinous agent, allowed a few minutes to completely clot, and then centrifuged. Serum is the supernatant obtained from whole blood that is allowed to clot (use red-top test tubes) and then centrifuged. Plasma is the supernatant resulting from whole blood that is collected into microtubes containing an anticoagulant (EDTA or heparin being the most common) and centrifuged. Blood with no anticoagulant may be used to perform a blood film immediately after collection via the cover-slip method (Jacobson 2007).
The best way to collect blood from an organism is when it is still alive. There are several methods used to collect blood samples, but care must be taken to ensure that the animal suffers minimal pain or stress during the procedure. If the animal will be alive for more than a few hours after a blood draw, the site should be cleansed before sampling using an aseptic technique such as 70% ethanol to prevent infection (Jacobson 2007). Blood may be drawn from the heart of a live animal only while the animal is under general anesthesia. Alternatively, blood can be drawn from the heart after the animal has been euthanized. Consult with the lab analyzing your samples for proper storage protocols.
In lizards, blood can be collected from several sites: the ventral tail vein, a toenail (following clipping) and the orbital sinus. Similarly, snake blood samples can also be obtained from the ventral tail vein and the heart (Jacobson 2007).
Parasites
Occasionally, parasites will be found in or on your study animal. Protozoan parasites are often found in the gastrointestinal tract and can be detected by performing an analysis on "fresh postmortem fecal samples" (Jacobson 2007). This can be accomplished through fecal flotation or fecal smears, except in the case of Cryptosporidium, which requires cytologic preparations of the intestinal or gastric mucosa. Due to the commensal nature of many protozoans, a histopathologic lesion is often the first indicator that an animal is infected (Jacobson 2007).
Metazoan (multicellular) parasites include endoparasites and ectoparasites. Endoparasites such as trematodes, nematodes, and cestodes can be found throughout the organ systems of reptiles. The Southeastern Cooperative Wildlife Disease Study suggests that ectoparasites and eggs from the same host may be preserved in a single vial in 70% isopropyl alcohol (personal communication December 21, 2012), and endoparasites can be preserved in 70% ethanol (USDA 2012). However, a consulting parasitologist may provide specialty preservatives for trematodes. It is also recommended to relax trematodes and cestodes in water for a few hours before fixation. If acanthocephalans are to be identified, they, too, must be soaked in water until their proboscis is extended (Jacobson 2007).
Skeletochronology
Bone samples can be used to age an animal using skeletochronology. According to Senning (1940) and Cagle (1946), amphibians and reptiles exhibit periodic growth, and this temporal pattern is recorded in certain bones. This pattern can also be observed in the epidermal scutes of chelonians (Halliday and Verrell 1988). Periods of growth are represented as broad bands of tissue—narrow lines are observed between broad bands and mark arrested growth (Halliday and Verrell 1988). These bands, or skeletal cyclic growth marks (SGMs), form annually and the age of the individual can be estimated by counting them (Buffrénil and Castanet 2000).
While studying the growth rates of Nile monitors (Varanus niloticus), Buffrénil and Castanet (2000) found that the fibula was the best bone to use for skeletochronological studies, and observing the ground sections of this bone under transmitted polarized light was the best way to count the growth marks. However, only a few studies have validated the idea that temperate species have a growth line for each year, and it is not known whether this method is also valid for tropical species due to a lack of data (Halliday and Verrell 1988). Halliday and Verrell (1988) also stated that the accuracy of the observers counting the SGMs could bring about different bone line counts for the same sample.
Euthanasia
The animal to be necropsied may be delivered dead or alive. If the animal is still alive, appropriate euthanasia methods must be considered.
Necropsies should be performed soon after the animal is euthanized to avoid autolysis. If the necropsy will not take place for a couple of hours, the reptile should be stored in a refrigerator. Depending on your purpose of study, specimens may also be stored in a freezer for days, weeks, or months before necropsy. Avoid freezing the animal before blood or tissue analysis, however, because it compromises samples (Stahl 1996).
Appropriate methods of euthanasia cause minimal stress and anxiety to the animal and initiate a rapid loss of consciousness before the arrest of cardiac, respiratory and brain function occurs (USDA 2007). Some euthanasia methods are outlined in Table 2.
Through discussions with veterinarians, as well as personal experience, we recommend the penetrating captive bolt or intravenous barbiturate solutions to euthanize reptiles. Both methods have the potential to alter histology and structure and modify the metabolic response of isolated tissues. Thus, pros and cons of each should be considered accordingly.
The following methods should not be used for killing reptiles: spinal cord severance, hypothermia, exsanguinations, chloroform, MS-222, ether, halothane, methoxyflurane, isoflurane, enflurane, carbon dioxide, neuromuscular blocking agents, ketamine hydrochloride, chloral hydrate, and procaine (Close and others 1997). While carbon dioxide is generally easily available and considered an acceptable method of euthanasia for certain species by the American Veterinary Medical Association, we do not recommend it as a first choice because reptiles are known to hold their breath for long periods of time and have a high tolerance of anoxia (Close and others 1997). Decapitation alone is also unacceptable, unless it is followed immediately by pithing of the brain (Close and others 1997).
Physical Euthanasia
A captive bolt pistol may be used to induce death by quickly destroying the brain. Warwick found that reptiles respond to stimuli after decapitation, suggesting consciousness. Reptiles thus require a method that quickly destroys the brain (Warwick 1990, cited by Close and others 1997). It is a recommended to restrain the animal in some way during physical euthanasia—both for personal safety and to ensure humane killing (Close and others 1997). A pair of snake tongs is an appropriate tool used to restrain the head and body of snakes and other reptiles.
Figure 2 illustrates the ideal location for captive bolt entry. The diameter, length of the bolt, and power of the charge used to dispatch the animal should be adjusted based on the size of the species.

Credit: Robin Bijlani
For tegus, use the scales highlighted in Figure 2. They are identified by finding the two frontal scales (analogous to a forehead) in the middle of the skull, between the eyes.
For snakes such as P. molurus bivittatus, use the back edge of the head and eyes to create guidelines to find the area to strike with the captive bolt (See Figure 2, right). Draw an imaginary line from the rear left of the head to the right eye and another line from the rear right of the head to the left eye. Position the captive bolt where those lines intersect. The bolt should enter at a slight angle toward the neck (approximately 60–70 degrees), not flush to the skull (Heard and Jacobson, personal conmmunication 2012).
Because the captive bolt will destroy the brain and skull of the animal, chemical euthanasia should be considered if any part of the head is to be collected or if examination of the mouth is desired.
Chemical Euthanasia
There are relatively few options for chemical euthanasia of reptiles (USDA 2007). Sodium pentobarbital is the preferred compound because of its ease of use, efficacy, and rapidity. When injected, sodium pentobarbital will suppress nerve impulses to the cortex and ultimately inhibit the respiratory system and kill the animal. However, sodium pentobarbital may cause the undesired effect of postmortem "kinking" at the injection site, which may create difficulty with collecting some data, such as body length (Conroy and others 2009).
Despite the benefits of chemical euthanasia, many of the options available for this purpose are controlled substances.
Equipment
- Sterile, nonporous surface to keep the necropsy area clean and sterile; a stainless steel table or surface covered in a plastic sheet is adequate
- Dissection kit including scalpels, scalpel blades, forceps, large and fine scissors
- Lab coat
- Goggles (or other eye protection)
- Latex or nitrile gloves
- Laboratory masks/face shields
- Closed-toed shoes
- Datasheet (see sample in Appendix)
- Pre-labeled plastic bags or plastic bottles with screw caps of various sizes for sample collection and storage; glass bottles are adequate although plastic is desired
- Spring scales for weighing samples and the animal
- Measuring stick or tape measure
- Permanent markers or pens
- Lighter or Bunsen burner for sterilizing instruments
- Vials with ethanol and formaldehyde for preserving parasites or tissue—ensureadequate ventilation
- Magnifying loops

Credit: Robin Bijlani
Methods
All the animal's basic information should be documented (identification number or name, age, sex, total length, snout-to-vent length, and weight) and thoroughly described. Length and weight measurements can further be used to calculate the body condition index, which is a useful tool to objectively assess nutritional condition (Jacobson 2007). The data collected should be as detailed as possible and include behavior observations, how and when the animal died, how the specimen was stored and for how long, etc. Sample datasheets can be found in the Appendix.
Examine every organ carefully to find abnormalities. Collect abnormal tissues and lesions, label them, and store them properly for further study. When describing your observations, take note of location, size, shape, textures, colors, smells, and overall appearance. It is better to take too many notes than to take too few. Photographs may also be useful for future reference.
External Examination
Before you make any incisions, examine the exterior of the animal and document any irregularities. Use a checklist incorporating the following elements to ensure your examination is complete:
- Skin: look for scars, irregular pigmentation, and wounds.
- Extremities: check for regenerated tail or missing limbs and digits.
- Ectoparasites: mites and ticks often hide in compact areas like the corners of the mouth, in and around the cloaca, the tympanic membrane, and the joints of the legs.
- Muscle condition
- Eyes: a sunken eye is a likely sign of severe dehydration or malnourishment.
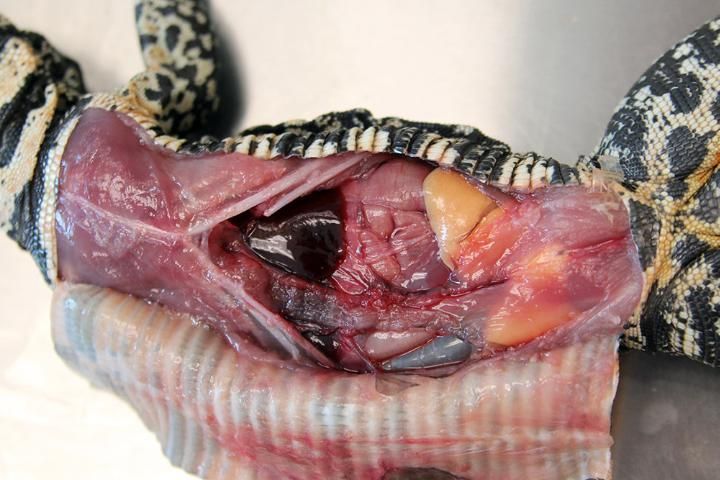
- Oral cavity: look for any signs of disease or inflammation. In reptiles, an infection (abscess) might appear like cottage cheese or curds, unlike any pus or liquid substance you would typically find in similar infections in mammalians. Inspect the teeth, trachea, and tongue. Abnormal fluid or material found on the roof of the mouth or choanal slit might be an indicator of an airway infection. Collect any lesions with adjacent normal tissue.
- Skeletal structure: the specimen should be carefully palpated for evidence of trauma, fractures, or other anomalies.
Dissection and Internal Examination
Lizards and snakes should be placed flat on their backs. Using a suitable cutting instrument (scalpel, scissors, knife), make an incision along the ventral midline, or belly, from the cloaca to just under the lower jaw (Jacobson 1978). A large vein, the ventral abdominal vein, is directly beneath the ventral midline. Shift the incision slightly to the left or right away from this vein to avoid excessive blood spill (Figure 4).
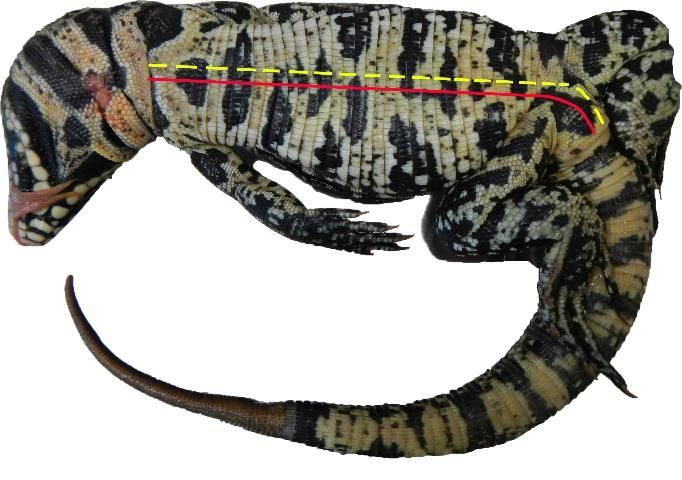
Credit: Michiko A. Squires
Turn back the skin and muscles on each side while cutting to reveal the body cavity (Figure 5). When you reach the rib cage, cut through the cartilage on one side of the sternum and pull back the ribs to see the heart and liver.
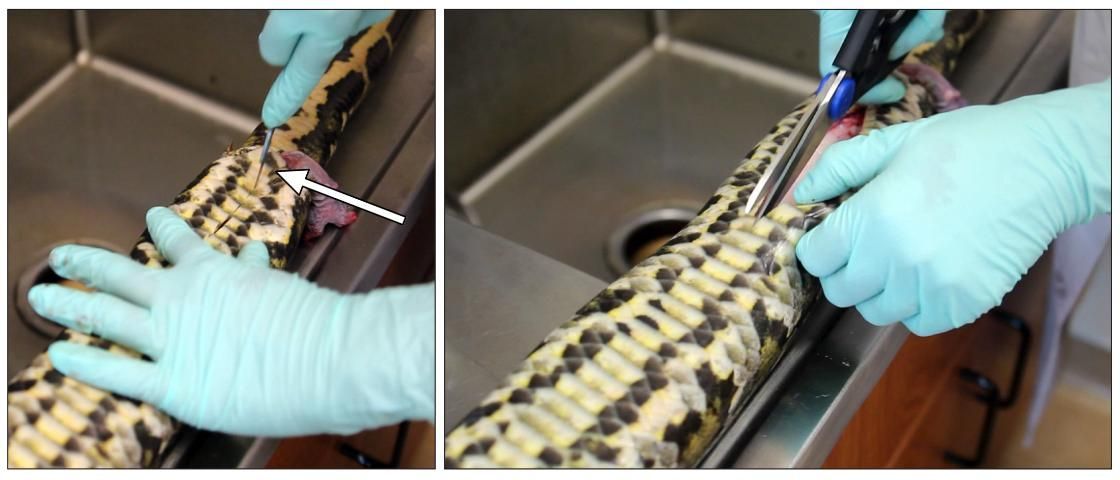
Credit: Robin Bijlani
Turtles and tortoises should also be laid on their backs using a molded wax block to secure the carapace. To free the plastron from the carapace, cut through the marginal bridge with a bone saw and free the ventral skin and muscle (Jacobson 1978).
On laterally compressed animals such as chameleons, perform the necropsy with the animal on its side (Figure 6). Dislocate the limbs of the chameleon by cutting through muscle and tendon groups underneath and fold them back out of the way. Continue by making an incision along the ventral midline from cloaca to forelimb and shoulder blade, up to the dorsal side of the animal. It may be necessary to cut some of the ribs to get the skin and body wall pulled back in order to view the body cavity. Following this procedure should enable you to avoid interference during the necropsy.
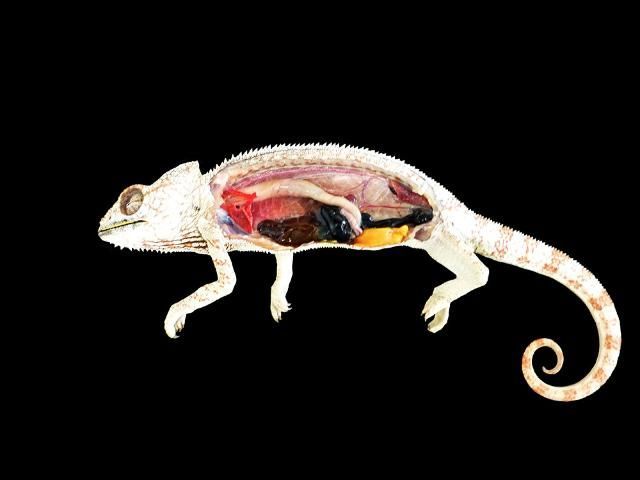
Credit: Sara E. Williams
The animal is now ready for internal examination (Figure 7).
Credit: Robin Bijlani
Fat Pads
The size and condition of a reptile's fat pads can be useful indicators of the body condition and diet of the animal (Figure 8). Large fat pads are a sign that the animal is finding a sufficient amount of food. Fat pads may also store environmental contaminants, so they should be removed, weighed, and labeled for further analysis. The arrangement of fat pads varies from species to species, even among those closely related. Usually they are found in the body cavity, and some fat bodies may also be present near the heart.
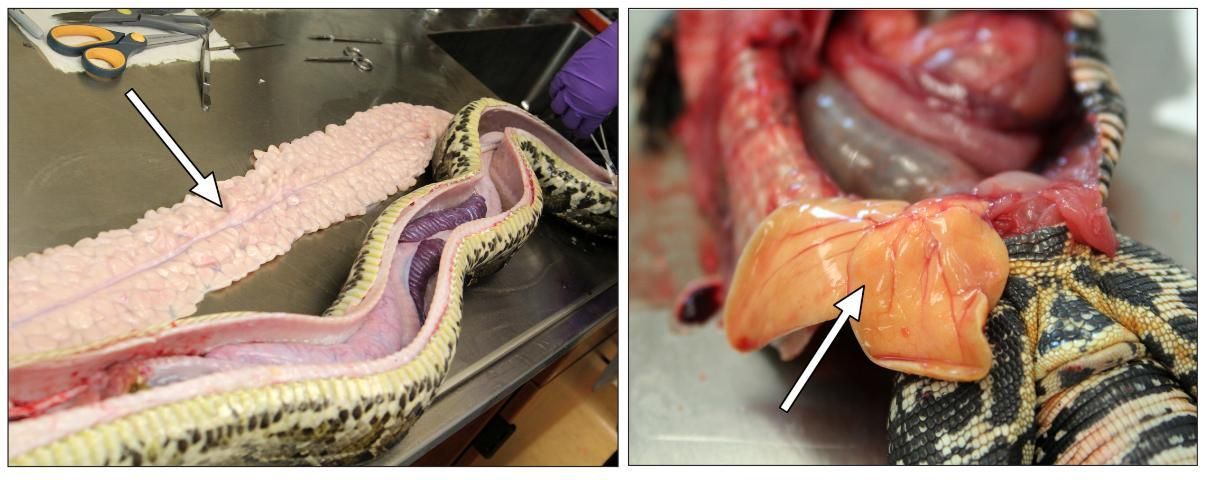
Credit: Robin Bijlani
Heart
Most reptiles have a three-chambered heart: two auricles and a ventricle, all surrounded by a pericardial membrane (Jacobson 2007). Carefully remove this membrane to observe the three chambers (Figure 9).
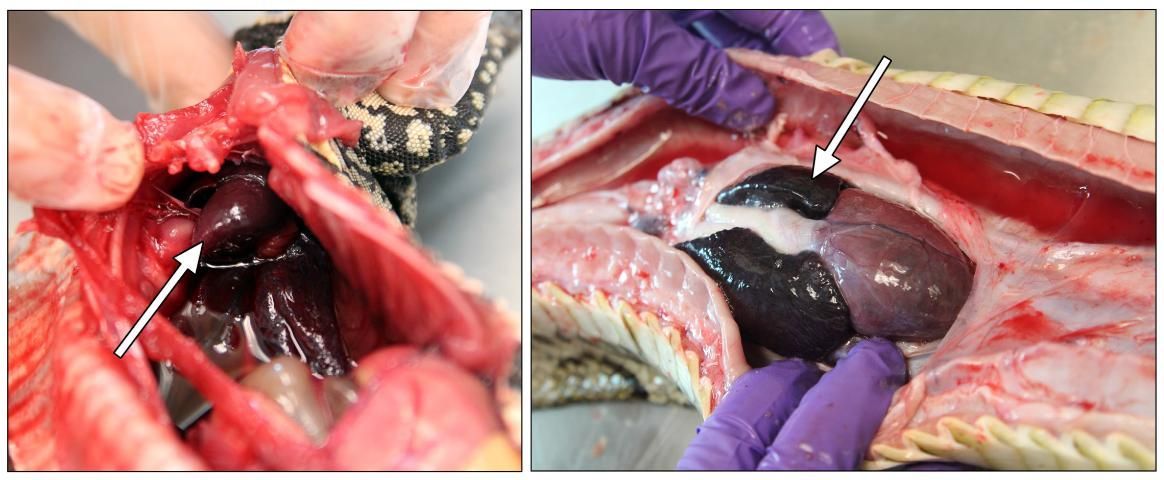
Credit: Robin Bijlani
Liver
A relatively large, lobed mahogany color or light brown organ just under the heart (Figure 10).

Credit: Robin Bijlani
Gallbladder
The gallbladder can usually be found near the pancreas and spleen, or, in some species, inside the liver. This organ contains bile that gives it a color in between green and yellow (Figure 11).
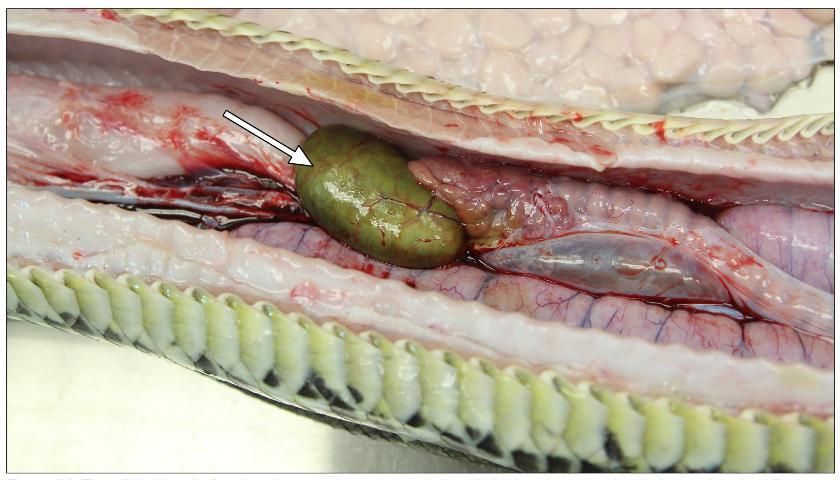
Credit: Robin Bijlani
Lungs
The lungs are located to the right and left of the esophagus and typically are a pinkish color. If a captive bolt was used for euthanasia, the lungs may have a dark red appearance with congested blood vessels. They should be palpated carefully to feel for any nodules or abnormalities. In addition, a small incision should be made to see if there are any parasites or exudates (Figure 12). The interior of healthy lungs has a honeycomb texture (Jacobson 2007).
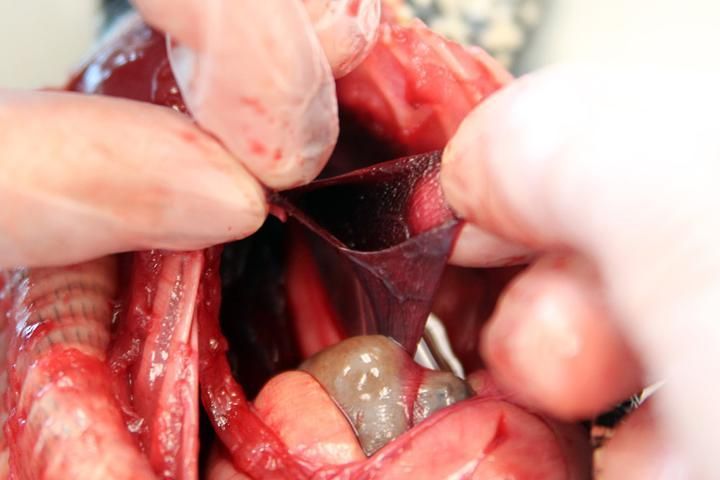
Credit: Robin Bijlani
Digestive System
The esophagus leads to the stomach. Next to the stomach is the pancreas, a light pink organ found on the smaller curvature of the stomach. In some species, the spleen is often in close association with the pancreas. The differentiation between the small and large intestine is by a demarcated dilation in intestinal diameter, and, in some species, by the location of the cecum (Figures 13 and 14).
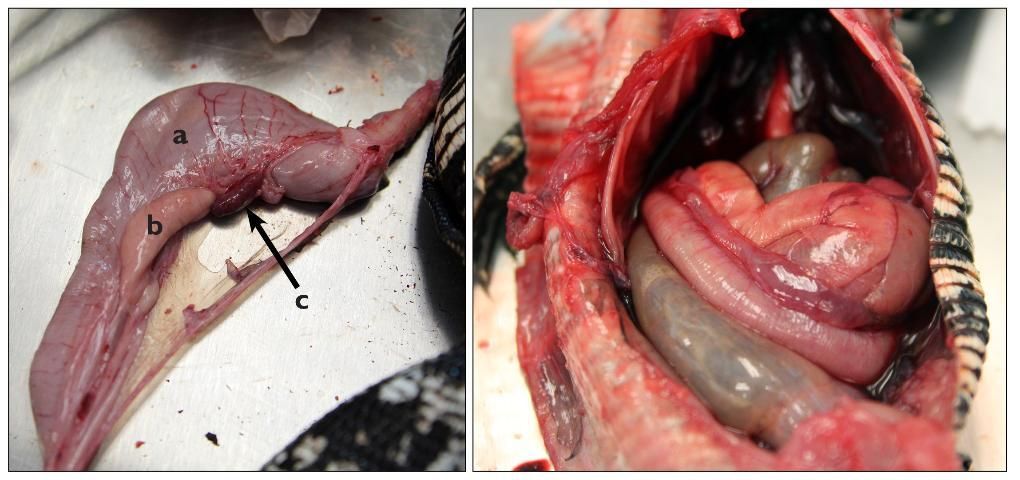
Credit: Robin Bijlani
Note: Chameleons may have blackened intestines. The color is natural pigmentation, and not abnormal (see Figure 6).
Remove the entire digestive tract (Figure 14) and weigh it with its contents. Afterwards, the gut contents can be removed, weighed separately, and stored for a short time in a freezer or in 5% formalin or 30–40% alcohol (Rabinowitz and others 2000) for further diet study. Collect gut contents either by cutting open the digestive tract or by simply squeezing the contents out of the tract and into a collection container through either the esophagus or the large intestine.
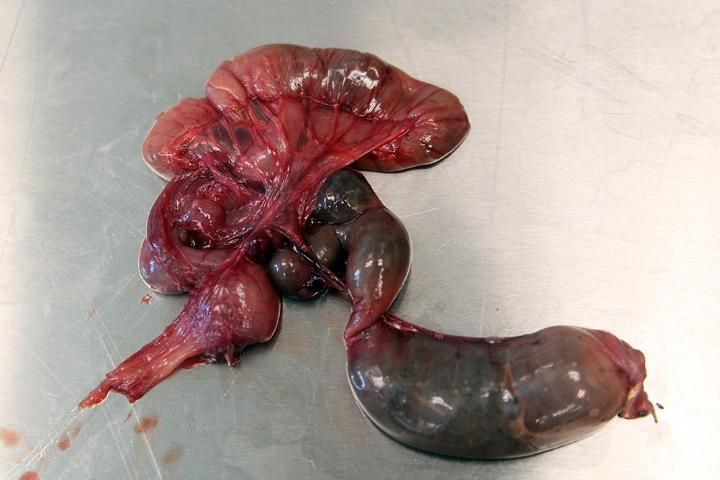
Credit: Robin Bijlani
Kidneys
The kidneys can be found at the posterior end of the reptile. You may need to cut the pelvis to remove them. In tegus, the kidneys are variant in color, usually light brown, oblong organs found on the left and right side. Green iguanas, monitor lizards, and snakes also have two kidneys, but they are dark brown and shaped like a stack of coins that has been tilted to the side (Figure 15).
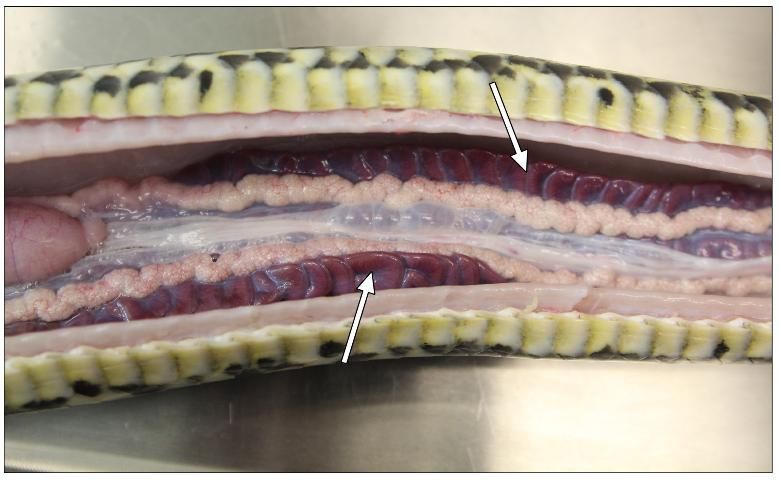
Credit: Robin Bijlani
Reproductive Organs and Adrenal Glands
After removing the intestinal tract, the ovaries or testicles are visible with the adrenal glands either dorsal or anterior to the kidneys. As seen in Figure 16, females will have a pair of ovaries and possibly follicles or eggs (left), and males have two testicles (right). The testes vary in size throughout the year.
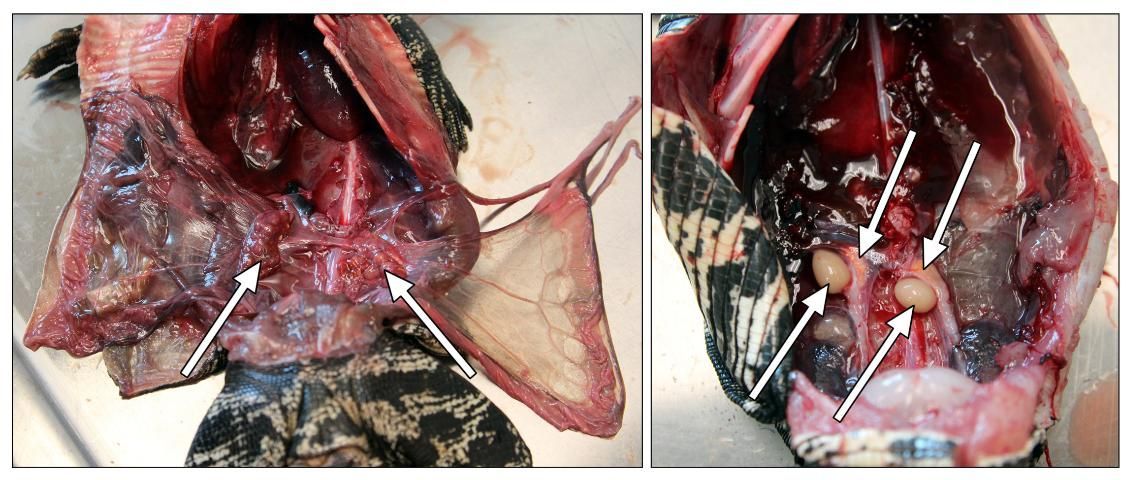
Credit: Robin Bijlani
The adrenal glands in reptiles are paired and can be found next to the reproductive organs. In tegus they are small, light orange, and grainy. In snakes they are long and tubular and can be either posterior or anterior to the gonads.
Further analysis and/or collection of the gonads or adrenal glands may be necessary, depending on the goal of the study. Increased stress hormones can cause microscopic changes in adrenal gland tissue, which may make it an important organ for examination.
Cleanup and Carcass Disposal
Immediately after the completion of the necropsy, put the animal in a plastic bag for storage and freeze or refrigerate it until it can be properly disposed of in a manner that is compliant with local agencies. The table, clothing, and equipment used should be first washed in hot water and detergent, then disinfected with a cleaning agent such as dilute sodium hypochlorite, chlorhexidine solution, or povidone iodine solution. These procedures will reduce cross-contamination and assure that the next necropsy and sample collection will be completed in a sterile environment. All disposable items used (scalpel blades, needles, and latex gloves) should be discarded appropriately in a biohazard or sharps container.
References
American Veterinary Medical Association. (2007, June). "AVMA Guidelines on Euthanasia: Formerly Report of the AVMA Panel on Euthanasia." https://www.avma.org/KB/Policies/Documents/euthanasia.pdf
de Buffrénil, V., Castanet, J. (2000). "Age Estimation by Skeletochronology in the Nile Monitor (Varanus niloticus), a Highly Exploited Species." Journal of Herpetology 34 (3): 414–424.
Cagle, F. R. (1946). The growth of the slider turtle, Pseudemys scripta elegans. American Midland Naturalist 36:685–729.
Canadian Council on Animal Care. (2004). "CCAC species-specific recommendations on: Amphibians and Reptiles." CCAC - Guidelines on amphibians and reptiles.pdf (no longer available online).
Close B., Banister, K., Baumans, V., Bernoth, E., Bromage, N. , Bunyan, J., Erhardt, W., Flecknell, P., Gregory, N., Hackbarth, H., Morton, D., and Warwick, C. (1997). "Recommendations for euthanasia of experimental animals: Part 2." Laboratory Animals 31: 1–32.
Conroy, C. J., Papenfuss, T., Parker, J., Hahn, N. E. (2009). "Use of Tricaine Methanesulfonate (MS222) for Euthanasia of Reptiles." Journal of the American Association of Laboratory Animal Science 48 (1): 28–32.
Halliday, T. R. and Verrell, P. A.. (1988). "Body Size and Age in Amphibians and Reptiles." Journal of Herpetology 22 (3): 253–265.
Hobson, K. A., Lisle Gibbs, H., and Gloutney, M. L. (1997). "Preservation of blood and tissue samples for stable-carbon and stable-nitrogen isotope analysis." Can. J. Zool. 75: 1720–1723.
Jacobson, E. R. (1978). "Reptile Necropsy Protocol." The Journal of Zoo Animal Medicine Vol. 9, No. 1: pp. 7–13.
Jacobson, E. R. (2007). Infectious Diseases and Pathology of Reptiles: Color Atlas and Text. Boca Raton: CRC Press. 219–235 and 304–305.
Jacobson, E. R. (2012). University of Florida. In discussion with the authors, March 2012.
Rabinowitz, A., Hart, J., White, L. (2000). Information from dead animals and their curation. In: Conservation Research in the African Rain Forests: A Technical Handbook. Pp. 191–201. White, L., Edwards, A. (eds). Wildlife Conservation Society, New York. ISBN: 0-9632064-4-3.
Raverty, S. A., and Gaydos, J. K. Killer Whale Necropsy and Disease Protocol. Retrieved from https://www.researchgate.net/publication/228877008_Killer_whale_necropsy_and_disease_testing_protocol
Senning, W. C. (1940), A study of age determination and growth of Necturus maculosus based on the parasphenoid bone. Am. J. Anat., 66: 483–495. doi: 10.1002/aja.1000660307
Seutin, G., White, B. N. and Boag, P. T. (1991). "Preservation of avian blood and tissue samples for DNA analysis." Can. J. Zool. 69: 82–90.
Stahl, S. (1996, February-March). "Necropsies: Post-Mortem Exams Help the Living." League of Florida Herp Societies. Retrieved from http://www.anapsid.org/necropsy.html
United States Department of Agriculture. "National Veterinary Services Laboratories Guidelines for Necropsy." 10 Sept. 2012. Retrieved from http://www.aphis.usda.gov/animal_health/lab_info_services/downloads/NecropsyGuideline.pdf
Vigil, S. Southeastern Cooperative Wildlife Disease Study, University of Georgia College of Veterinary Medicine. In discussion with the author, December 21, 2012.
Appendix
Appendix A: Example Animal Intake Form

Credit:
Appendix B: Example Necropsy Data Collection Form

Credit:

Credit:
Table 1. Summary of common samples for analysis. Confer with diagnostic laboratory for specific requirements.
Table 2. Acceptable and Unacceptable Euthanasia Methods. This table is based on information provided by the AVMA Guidelines for Euthanasia 2007.