This publication is aimed primarily at deer farmers raising white-tailed deer in Florida. However, the knowledge shared in this piece can also prove valuable to deer farmers across the nation who might face potential occurrences of deerpox. Furthermore, the insights provided here can be of assistance to wildlife deer managers who may come across situations involving young fawn deer cases.
Pox viruses are widespread in the animal world and infect a large number of hosts, including insects, reptiles, birds, and many mammal species. While some pox viruses like smallpox virus and chicken pox virus are highly adapted to humans, some, like monkeypox virus, can be transmitted from species to species. All pox viruses are highly contagious and usually result in lesions or rashes on the skin. Poxvirus infections occur in domestic hoofstock including cattle, sheep, goats, camels, horses, and swine. In addition, poxvirus infections have been reported in wild ungulates including mountain sheep, mountain goats, reindeer, mule deer, musk-ox, caribou, moose, and white-tailed deer.
In 1983, a genetically distinct pox virus was isolated from two skin lesions from free-ranging mule deer from Wyoming (the virus species Mule deerpox virus). Since the 1990s and early 2000s, several cases of the disease mule deerpox virus have been reported from black-tailed deer from California and Oregon and a white-tailed deer from Mississippi, suggesting that this virus may be a potential emerging pathogen for white-tailed deer. It remains unclear whether the emergence of this virus is due to increased prevalence or because of increased detection.
Why is mule deerpox virus important for us here in Florida?
In 2016, the UF/IFAS Cervidae Health Research Initiative (CHeRI) was presented with a case of a four-week-old farmed white-tailed deer fawn that died from a secondary bacterial infection but had lesions consistent with a pox virus infection. By culturing a swab of the lesion, we were able to grow and then sequence the whole genome of a virus that we identified as Mule Deerpox Virus. The genome sequence was 100% identical to the one found in two Wyoming mule deer, 99% similar to a case from a Mississippi white-tailed deer, and 98% similar to virus from black-tailed deer from California and Oregon. Since that first report, CHeRI has identified the virus in deer from multiple farms. Cases have typically presented as fawns fewer than 3 months of age with skin lesions that lead to secondary bacterial infections. Cases have been found throughout Florida.
How is deerpox transmitted?
Pox viruses are highly contagious. Pox viruses can be transmitted by aerosols (small saliva particles in the air) or by direct contact among sick animals. Virus particles are shed from skin lesions and ocular and nasal discharges during the acute stage of the disease, contaminating equipment and housing facilities and allowing the virus to spread from animal to animal. Biting insects (mosquitos and biting flies) or rodents may also transmit the virus, as occurs with other pox viruses. More research on transmission or potential wild hosts is warranted.
What are the signs of pox virus in deer?
Clinical illness from deerpox virus seems to be more common in fawns, primarily during the warm months of the year. The incubation period for deerpox virus in white-tailed deer is still unclear; however, it is thought to be from one to two weeks. The disease generally involves the development of vesicles, or blisters on the skin that burst and develop into ulcers with a crust along the edge of the lesion. These lesions commonly can be seen on the neck and mucocutaneous tissues of the head (eyelids, lips, ear tips, nose, hooves, and surrounding area). Other gross lesions reported in some cases include keratoconjunctivitis (combined inflammation of the cornea and conjunctiva of the eye) and hair loss in affected areas. In addition, ulcers can form on the upper alimentary tract (lips, mouth, tongue, esophagus); in stomachs (reticulum and rumen); and on the teats. Symptoms such as weakness and weight loss have also been described.
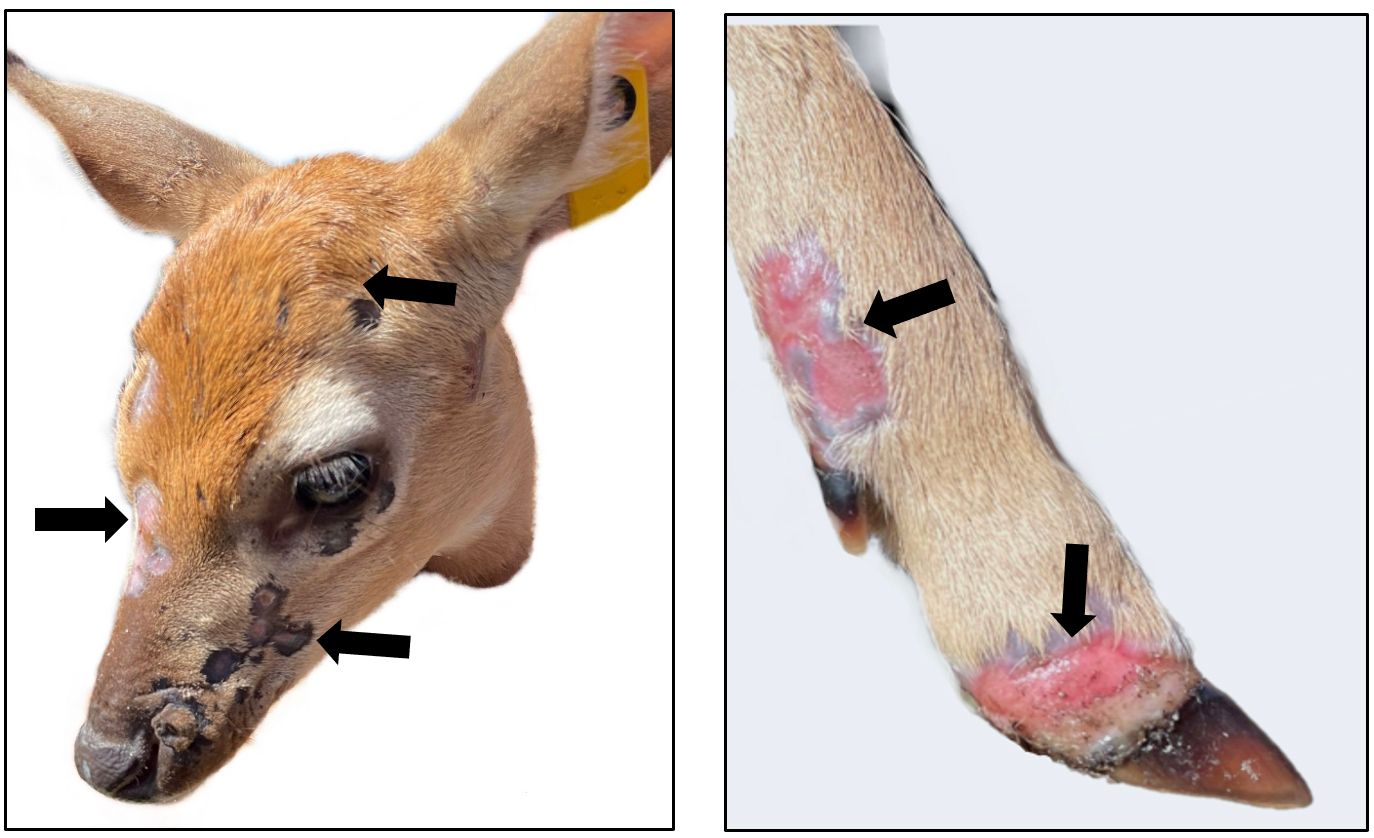
Credit: UF/IFAS CHeRI
Can deerpox kill deer?
If a deerpox infection affects the entire body, it can be fatal. However, in most cases, the disease has not been fatal. Infected deer that do die with deerpox usually die of a secondary bacterial infection or the pain of alimentary ulcers makes them unable to eat and internal ulcers interfere with digestion, and they starve to death. At this point, it is unclear if animals that recover from deerpox become immune for the rest of their lives to subsequent deerpox virus reinfection.
How is deerpox virus diagnosed?
Poxvirus infections are diagnosed based on clinical manifestation (appearance of the characteristic skin lesions); however, identifying deerpox cases can sometimes be challenging if the animal only develops internal lesions. In these cases, clinical manifestation may be lethargy and decreased appetite. However, PCR-based detection of viral DNA from a lesion swab or tissue sample is recommended. If you suspect you have a deerpox case on your farm, identify the infected animal and quickly isolate it from the rest of your herd. Because mule deerpox virus is highly contagious, outbreaks can occur on your farm. If you suspect pox virus in an animal, the best way to rule it out is by giving the CHeRI team a call. We will swab lesions of live animals to detect DNA from the virus that causes the disease. Rapid and accurate diagnosis can prevent a farm-wide outbreak of the virus.
How is deerpox treated?
At present, there are no vaccines available and no specific treatment for deerpox, other than supportive care to prevent malnutrition, dehydration, and secondary bacterial infections.
Can people catch deerpox?
Mule deerpox virus has not been observed to be transmitted to people; however, other pox viruses can be transmitted to people. If you spot lesions on your farmed deer, wear proper personal protection. Wear long sleeves and gloves to protect your skin from open lesions, and properly dispose of or wash clothing that has been in contact with potentially infected deer. Always wash your hands after handling deer.
Can deerpox affect other livestock?
Deerpox virus is only known to affect farmed and wild cervids and is not known to be contagious towards other livestock species.
How can I prevent deerpox from spreading in my herd?
If you suspect a case on your deer farm, particularly if it is among your fawns, separate the suspected animals and prevent contact of infected animals with healthy ones. Thoroughly disinfect equipment, tools, and facilities. The virus is relatively resistant to physical and chemical cleaning. To disinfect surfaces, use sodium hydroxide solution (0.8%), sodium hypochlorite (bleach) at 1%, quaternary ammonium compounds, and detergents. Always read the label carefully and follow all instructions for safe handling and use of products. Alcohol is not a suitable disinfectant. Make sure to wash and disinfect your hands and any clothing or shoes after caring for animals that may be infected. Before introducing new deer to your herd, ensure the new animals do not have any visible lesions or any other signs of disease. Currently, we do not know if pests such as rodents or insects can spread deerpox virus; however, it is always important to implement a comprehensive pest-control program on your farm. If you have questions, please feel free to contact Dr. Juan Campos Krauer for a farm consultation or the CHeRI hotline (352-562-3337) if you would like diagnostic services for sick or deceased deer. CHeRI’s mission is to improve the health of Florida’s farmed deer and the productivity of the Florida farmed deer industry.
UF/IFAS Cervidae Health Research Initiative: https://wec.ifas.ufl.edu/cheri/
Resources Consulted
Adams, M. J., E. J. Lefkowitz, A. M. Q. King, B. Harrach, R. L. Harrison, N. J. Knowles, et al. 2017. “Changes to Taxonomy and the International Code of Virus Classification and Nomenclature Ratified by the International Committee on Taxonomy of Viruses.” Archives of Virology 162:2505–2538. https://doi.org/10.1007/s00705-017-3358-5
Afonso, C. L., G. Delhon, E. R. Tulman, Z. Lu, A. Zsak, V. M. Becerra, L. Zsak, G. F. Kutish, and D. L. Rock. 2005. “Genome of Deerpox Virus.” Journal of Virology 79:966–977. https://doi.org/10.1128/JVI.79.2.966-977.2005
Baughman, B., S. Zhang, L. Jin, L. W. Pace, J. Cooley, L. Yan, and M. Z. Zhang. 2011. “Diagnosis of Deerpox Virus Infection in a White-Tailed Deer (Odocoileus virginianus) fawn.” Journal of Veterinary Diagnostic Investigation 23:965–970. https://doi.org/10.1177/1040638711416621
Dieterich, R. A., G. R. Spencer, D. Burger, A. M. Gallina, and J. VanderSchalie. 1981. “Contagious Ecthyma in Alaskan Musk-Oxen and Dall Sheep.” Journal of the American Veterinary Medical Association 179:1140–1143.
Gillespie, J. H., and J. F. Timoney. 1981. Hagan and Bruner’s Infectious Diseases of Domestic Animals. Cornell University Press, Ithaca, New York. Pp. 527–550.
Junge, R. E., M. C. Duncan, E. Miller, D. Gregg, and M. Kombert. 2003. “Clinical Presentation and Antiviral Therapy for Poxvirus Infection in Pudu (Pudu puda).” Journal of Zoo and Wildlife Medicine 31:412–418. https://doi.org/10.1638/1042-7260(2000)031[0412:CPAATF]2.0.CO;2
Kummuneje, K., and J. Krogsrud. 1979. “Contagious Ecthyma (orf) in Reindeer (Rangifer tarandus tarandus).” Veterinary Record 105:60–61.
Moerdyk-Schauwecker, M., K. Eide, R. Bildfell, R. J. Baker, W. Black, D. Graham, K. Thompson, G. Crawshaw, G. F. Rohrmann, and L. Jin. 2009. “Characterization of Cervidpoxvirus Isolates from Oregon, California and Eastern Canada.” Journal of Veterinary Diagnostic Investigation 21:487–492. https://doi.org/10.1177/104063870902100409
Patton, J. F., R. W. Nordhausen, L. W. Woods, and N. J. MacLachlan. 1996. “Isolation of a Poxvirus from a Black-Tailed Deer (Odocoileus hemionus columbianus).” Journal of Wildlife Diseases 32:531–533. https://doi.org/10.7589/0090-3558-32.3.531
Quinn, P. J., B. K. Markey, F. C. Leonard, P. Hartigan, S. Fanning, and E. S. Fitzpatrick. 2011. Veterinary Microbiology and Microbial Disease, 2nd Edition, 593–602. Oxford: Wiley-Blackwell.
Samuel, W. M., G. A. Chalmers, J. G. Stelfox, A. Loewen, and J. J. Thomsen. 1975. “Contagious Ecthyma in Bighorn Sheep and Mountain Goats in Western Canada.” Journal of Wildlife Diseases 11:26–30. https://doi.org/10.7589/0090-3558-11.1.26
Sayler, K. A, K. Subramaniam, J. M. Jacob, J. C. Loeb, W. F. Craft, L. L. Farina, N. I. Stacy, et al. “Characterization of mule deerpox virus in Florida White-Tailed Deer Fawns Expands the Known Host and Geographic Range of This Emerging Pathogen.” Archives of Virology. September 2018. https://doi.org/10.1007/s00705-018-3991-7
Skinner, M. A., R. M. Buller, I. K. Damon, E. J. Lefkowitz, G. McFadden, C. J. McInnes, A. A. Mercer, R. W. Moyer, and C. Upton. 2012. “Poxviridae” In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses, edited by A. M. Q. King, M. J. Adams, E. B. Carstens, and E. J. Lefkowitz, 291–309. New York: Elsevier Academic Press. https://doi.org/10.1016/B978-0-12-384684-6.00028-8
Smith, T. C., W. E. Heimer, and W. J. Foreyt. 1982. “Contagious Ecthyma in an Adult Dall Sheep (Ovis dalli dalli) in Alaska.” Journal of Wildlife Diseases 18:111–112. https://doi.org/10.7589/0090-3558-18.1.111
Upton, C., S. Slack, A. L. Hunter, A. Ehlers, and R. L. Roper. 2003. “Poxvirus Orthologous Clusters: Toward Defining the Minimum Essential Poxvirus Genome.” Journal of Virology 77:590–600. https://doi.org/10.1128/JVI.77.13.7590-7600.2003
Williams, E. S., V. M. Becerra, T. J. Graham, M. J. Owens, and C. E. Nunamaker. 1985. “Spontaneous Poxviral Dermatitis and Keratoconjunctivitis in Free-Ranging Mule Deer (Odocoileus hemionus) in Wyoming.” Journal of Wildlife Diseases 21:430–433. https://doi.org/10.7589/0090-3558-21.4.430