The transition to lactation (3 weeks before to 3 weeks after calving) is a challenging period for a high producing dairy cow. This period is characterized by a decrease in dry-matter intake (DMI), leading to a sharp decrease in glucose and calcium, and an increase in body fat mobilization in the form of non-esterified fatty acids (NEFA). This results in products such as beta-hydroxybutyrate (BHBA) accumulating from incomplete oxidation of NEFA (Vazquez-Añon et al. 1994).
Neutrophils (PMN) are the main leukocyte type involved in clearing bacteria after uterine infection (Hussain 1989; Gilbert et al. 2007); however, during the period of negative energy balance, dairy cows experience a reduction in PMN function, including reduced phagocytosis and killing capacity (Cai et al. 1994; Kehrli and Goff 1989; Gilbert et al. 1993). The factors that account for such reduction include decreased PMN glycogen stores, decreased blood calcium concentration, and increased NEFA and BHBA. In particular, cows that develop uterine disease have a more pronounced decrease in DMI (Huzzey et al. 2007), an increase in NEFA and BHBA, and a decrease in blood PMN pathogen phagocytosis (Kim et al. 2005) and killing (Hammon et al. 2006). In the study by Hammon et al. (2006), NEFA was negatively associated with PMN oxidative burst activity. Recently, BHBA was also observed to be negatively associated with PMN phagocytosis, extracellular trap formation, and killing of bacteria (Grinberg et al. 2008).
Neutrophils rely on different glucose sources for different functions. They depend mainly on extracellular glucose (but can use glycogen under hypoglycemic conditions) for the energy required for chemotaxis, while they depend mainly on intracellular glycogen and glycogenolysis for the glucose necessary for phagocytosis and killing (Kuehl and Egan 1980; Weisdorf et al. 1982a; Weisdorf et al. 1982b). Whereas chemotactic stimuli (such as FMLP, C5a, and aracdonic acid) accelerate glucose uptake, phagocytic stimuli (such as opsonized zymosan particles) failed to increase glucose uptake but increased glycogen breakdown (Weisdorf et al. 1982a; Weisdorf et al. 1982b). Therefore, the low glucose concentrations observed in the first 10 days of lactation (Vazquez-Añon et al. 1994) might directly impair PMN chemotaxis and could lead to decreased PMN glycogen stores. In turn, the impaired PMN chemotaxis and decreased PMN glycogen stores lead to decreased phagocytic and killing capability (and possibly chemotaxis), which would predispose cows to disease. In a recent study, PMN glycogen stores were found to be reduced early postpartum and such reduction was more pronounced in cows that developed uterine disease (Galvão et al. 2010a). Calcium is an important second messenger for PMN activation. In a recent study (Figure 1), cows that developed uterine disease had a greater reduction in calcium concentration than healthy cows (Martinez-Patino et al. 2011).
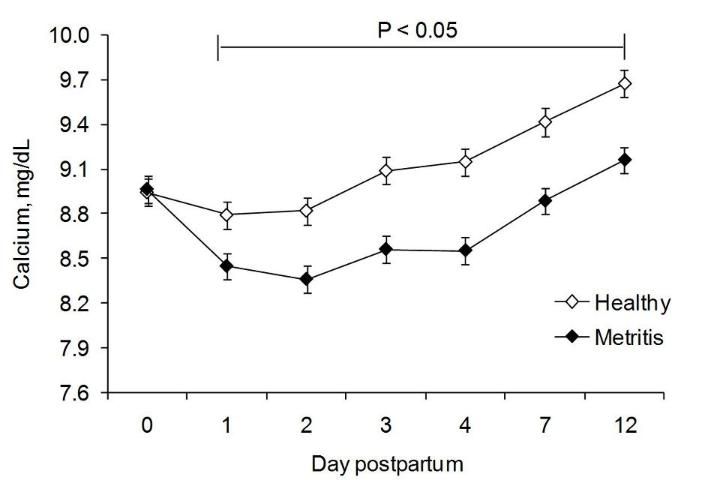
Credit: Martinez-Patino et al. (2011).
The high concentrations of cortisol and estradiol are also believed to contribute to the overall state of immunosuppression around calving (Goff and Horst 1997). If the immune system is not able to eliminate bacterial infection, disease is established. Early postpartum (< 21 days in milk, or DIM) cows are affected with metritis (severe and acute). Some cows clear the infection but others remain chronically infected (> 21 DIM), and the condition is called endometritis. Regardless of the condition, the overall effect of uterine infection is damage to the endometrium and activation of inflammation with release of pro-inflammatory cytokines, including tumor necrosis factor-α (TNFα), interleukin-1 (IL-1), and interleukin-6 (IL-6), and chemokines, including interleukin-8 (IL-8) (Chapwanya et al. 2009; Galvão et al. 2011). Damage to the endometrium is caused by the bacteria and by neutrophils releasing proteolytic granules and reactive oxygen species.
Pathogenic bacteria associated with metritis and endometritis are Escherichia coli, Arcanobacterium pyogenes, Fusobacterium necrophorum, and Prevotella maleninogenicus. Infection with these pathogenic bacteria will induce the release of pro-inflammatory cytokines such as TNFα, which have been found to stimulate the release of prostaglandin-F2α (PGF2α) from the endometrium and luteal cells and to induce luteolysis (Skarzynski et al. 2005; Kaneko and Kawakami 2008; Kaneko and Kawakami 2009). On the other hand, IL-1 and IL-6 have been found to decrease the expression of oxytocin receptors in endometrial cells, which could impair the mechanism of luteolysis (Leung et al. 2001). Therefore, inflammation could have a bimodal effect on the length of the estrus cycle.
Although the initial response to infection might be to lyse the corpus luteum (CL), another response observed in cows having uterine disease is a prolonged luteal phase. Prolonged luteal lifespan was observed when the first ovulation postpartum occurred in the presence of a heavily contaminated uterus (Olson et al. 1984), or when A. pyogenes was infused into the uterine lumen (Farin et al. 1989). Escherichia coli releases the endotoxin lipopolysaccharide (LPS), which impairs the release of both gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) (Peter et al. 1989), decreases aromatase activity (Herath et al. 2009), and increases the prostaglandin-E2 (PGE) to PGF2α ratio (Herath et al. 2009). The consequences of decreased aromatase activity are decreased follicular growth and estradiol production (Williams et al. 2007; Williams et al. 2008). Decreased GnRH/LH release leads to decreased ovulation rate (Peter et al. 1989), and an increase in the ratio of PGE to PGF2α leads to extended luteal phase when ovulation does occur (Farin et al. 1989). A. pyogenes releases the cholesterol-dependent cytotoxin pyolysin, which damages the endometrium and leads to the production of pro-inflammatory cytokines, decreased endometrial oxytocin receptors, and impairment of embryo development (Hansen et al. 2004; Hill and Gilbert 2008). The combined effect of bacterial infection and activation of inflammation is damage to the endometrium and embryo, delayed ovulation, shortened or extended luteal phase after ovulation, increased time to first insemination, decreased conception rates, increased time to conception, and increased pregnancy loss (Opsomer 2000; Galvão et al. 2009; Galvão et al. 2010b).
In summary, PMN are the main leukocyte type involved in clearing bacteria after uterine infection; however, during the period of negative energy balance, dairy cows experience a reduction in PMN function, including reduced phagocytosis and killing capacity. This reduction is more pronounced in cows that develop uterine disease. Glycogen is the main source of energy for PMN phagocytosis and killing; calcium is an important second messenger for PMN activation; NEFA is associated with impaired PMN oxidative burst activity; and BHBA reduces PMN phagocytosis, extracellular trap formation, and killing of bacteria. If the immune system is not able to eliminate bacterial infection, disease is established. The combined effect of bacterial infection and activation of inflammation is damage to the endometrium and embryo, delayed ovulation, shortened or extended luteal phase after ovulation, increased time to first insemination, decreased conception rates, increased time to conception, and increased pregnancy loss.
References
Cai, T.Q., P.G. Weston, L.A. Lund, B. Brodie, D.J. McKenna, and W.C. Wagner. 1994. "Association between Neutrophil Functions and Periparturient Disorders in Cows." Am. J. Vet. Res. 55: 934–43.
Chapwanya, A., K.G. Meade, M.L. Doherty, J.J. Callanan, J.F. Mee, and C. O'Farrelly. 2009. "Histopathological and Molecular Evaluation of Holstein-Friesian Cows Postpartum: Toward an Improved Understanding of Uterine Innate Immunity." Theriogenology 71: 1396–407.
Farin, P.W., L. Ball, J.D. Olson, R.G. Mortimer, R.L. Jones, W.S. Adney, and A.E. McChesney. 1989. "Effect of Actinomyces Pyogenes and Gram-negative Anaerobic Bacteria on the Development of Bovine Pyometra." Theriogenology 31: 979–89.
Galvão, K.N., L.F. Greco, J.M. Vilela, M.F. Sá Filho, and J.E. Santos. 2009. "Effect of Intrauterine Infusion of Ceftiofur on Uterine Health and Fertility in Dairy Cows." J. Dairy Sci. 92: 1532–42.
Galvão, K.N., M.J. Flaminio, S.B. Brittin, R. Sper, M. Fraga, L. Caixeta, A. Ricci, C.L. Guard, W.R. Butler, and R.O. Gilbert. 2010a. "Association between Uterine Disease and Indicators of Neutrophil and Systemic Energy Status in Lactating Holstein Cows." J. Dairy Sci. 93: 2926–37.
Galvão, K.N., M. Frajblat, W.R. Butler, S.B. Brittin, C.L. Guard, and R.O. Gilbert. 2010b. "Effect of Early Postpartum Ovulation on Fertility in Dairy Cows." Reprod. Domest. Anim. 45: e207–11.
Galvão, K.N., N.R. Santos, J.S. Galvão, and R.O. Gilbert. 2011. "Association between Endometritis and Endometrial Cytokine Expression in Postpartum Holstein Cows." Theriogenology 76: 290–9.
Gilbert, R.O., Y.T. Gröhn, P.M. Miller, and D.J. Hoffman. 1993. "Effect of Parity on Periparturient Neutrophil Function in Dairy Cows." Vet. Immunol. Immunopathol. 36: 75–82.
Gilbert, R.O., N.R. Santos, K.N. Galvão, S.B. Brittin, and H.B. Roman. 2007. "The Relationship between Postpartum Uterine Bacterial Infection (BI) and Subclinical Endometritis (SE)." J. Dairy Sci. 90(Suppl. 1): 469 (Abstr).
Goff, J.P., and R.L. Horst. 1997. "Physiological Changes at Parturition and Their Relationship to Metabolic Disorders." J. Dairy Sci. 80: 1260–8.
Grinberg, N., S. Elazar, I. Rosenshine, and N.Y. Shpigel. 2008. "Beta-hydroxybutyrate Abrogates Formation of Bovine Neutrophil Extracellular Traps and Bactericidal Activity against Mammary Pathogenic Escherichia Coli." Infect. Immun. 76: 2802–7.
Hammon, D.S., I.M. Evjen, T.R. Dhiman, J.P. Goff, and J.L. Walters. 2006. "Neutrophil Function and Energy Status in Holstein Cows with Uterine Health Disorders." Vet. Immunol. 113: 21–9.
Hansen, P.J., P. Soto, and R.P. Natzke. 2004. "Mastitis and Fertility in Cattle Possible-involvement of Inflammation or Immune Activation in Embryonic Mortality." Am. J. Reprod. 60: 1104–9.
Herath, S., S.T. Lilly, D.P. Fischer, E.J. Williams, H. Dobson, C.E. Bryant, and I.M. Sheldon. 2009. "Bacterial Lipopolysaccharide Induces an Endocrine Switch from Prostaglandin F2alpha to Prostaglandin E2 in Bovine Endometrium." Endocrinology 150: 1912–20.
Hill, J., and R. Gilbert. 2008. "Reduced Quality of Bovine Embryos Cultured in Media Conditioned by Exposure to an Inflamed Endometrium." Aust. Vet. J. 86: 312–6.
Hussain, A.M. 1989. "Bovine Uterine Defense Mechanism: A Review." J. Vet. Med. B. 36: 641–51.
Huzzey, J.M., D.M. Veira, D.M. Weary, and M.A. von Keyserlingk. 2007. "Prepartum Behavior and Dry Matter Intake Identify Dairy Cows at Risk for Metritis." J. Dairy Sci. 90: 3220–33.
Kaneko, K., and S. Kawakami. 2008. "Influence of Experimental Intrauterine Infusion of Arcanobacterium Pyogenes Solution on Ovarian Activity in Cycling Cows." J. Vet. Med. Sci. 70: 77–83.
Kaneko, K., and S. Kawakami. 2009. "The Roles of PGF (2alpha) and PGE (2) in Regression of the Corpus Luteum after Intrauterine Infusion of Arcanobacterium Pyogenes in Cows." Theriogenology 71: 858–63.
Kehrli, M.E. Jr., and J.P. Goff. 1989. "Periparturient Hypocalcemia in Cows: Effects on Peripheral Blood Neutrophil and Lymphocyte Function." J. Dairy Sci. 72: 1188–96.
Kim, I.H., K.J. Na, and M.P. Yang. 2005. "Immune Responses during the Peripartum Period in Dairy Cows with Postpartum Endometritis." J. Reprod. Dev. 51: 757–64.
Kuehl, F.A. Jr., and R.W. Egan. 1980. "Prostaglandins, Arachidonic Acid, and Inflammation." Science 210: 978–84.
Leung, S.T., Z. Cheng, E.L. Sheldrick, K. Derecka, K. Derecka, A.P. Flint, and D.C. Wathes. 2001. "The Effects of Lipopolysaccharide and Interleukins-1 alpha, -2 and -6 on Oxytocin Receptor Expression and Prostaglandin Production in Bovine Endometrium." J. Endocrinol. 168: 497–508.
Martinez-Patino, N., C.A. Risco, F. Maunsell, K.N. Galvão, and J.E. Santos. 2011. Presentation at the 44th Annual Conference of the American Association of Bovine Practitioners, St. Louis, Missouri, September 22–24, 2011.
Olson, J.D., L. Ball, R.G. Mortimer, P.W. Farin, W.S. Adney, and E.M. Huffman. 1984. "Aspects of Bacteriology and Endocrinology of Cows with Pyometra and Retained Fetal Membranes." Am. J. Vet. Res. 45: 2251–5.
Opsomer, G., Y.T. Gröhn, J. Hertl, M. Coryn, H. Deluyker, A. de Kruif. 2000. "Risk Factors for Post-partum Ovarian Dysfunction in High Producing Dairy Cows in Belgium: A Field Study." Theriogenology 53(4): 841–57.
Peter, A.T., W.T. Bosu, and R.J. DeDecker. 1989. "Suppression of Preovulatory Luteinizing Hormone Surges in Heifers after Intrauterine Infusions of Escherichia Coli Endotoxin." Am. J. Vet. Res. 50: 368–73.
Sheldon, I.M., and H. Dobson. 2004. "Postpartum Uterine Health in Cattle." Anim. Reprod. Sci. 82-83: 295–306.
Skarzynski, D.J., J.J. Jaroszewski, and K. Okuda. 2005. "Role of Tumor Necrosis Factor-alpha and Nitric Oxide in Luteolysis in Cattle." Domest. Anim. Endocrinol. 29: 340–6.
Vazquez-Añon, M., S. Bertics, M. Luck, R.R. Grummer, J. Pinheiro. 1994. "Peripartum Liver Triglyceride and Plasma Metabolites in Dairy Cows." J. Dairy Sci. 77: 1521–8.
Weisdorf, D.J., P.R. Craddock, and H.S. Jacob. 1982a. "Glycogenolysis versus Glucose Transport in Human Granulocytes: Differential Activation in Phagocytosis and Chemotaxis." Blood. 60: 888–93.
Weisdorf, D.J., P.R. Craddock, and H.S. Jacob. 1982b. "Granulocytes Utilize Different Energy Sources for Movement and Phagocytosis." Inflammation 6: 245–56.
Williams, E.J., D.P. Fischer, D.E. Noakes, G.C. England, A. Rycroft, H. Dobson, and I.M. Sheldon. 2007. "The Relationship between Uterine Pathogen Growth Density and Ovarian Function in the Postpartum Dairy Cow." Theriogenology 68: 549–59.
Williams, E.J., K. Sibley, A.N. Miller, E.A. Lane, J. Fishwick, D.M. Nash, S. Herath, G.C. England, H. Dobson, and I.M. Sheldon. 2008. "The Effect of Escherichia Coli Lipopolysaccharide and Tumour Necrosis Factor Alpha on Ovarian Function." Am. J. Reprod. Immunol. 60: 462–73.