The dairy cow is unique because virtually all cows are infected with bacteria right after calving (Sheldon and Dobson 2004; Figure 1).
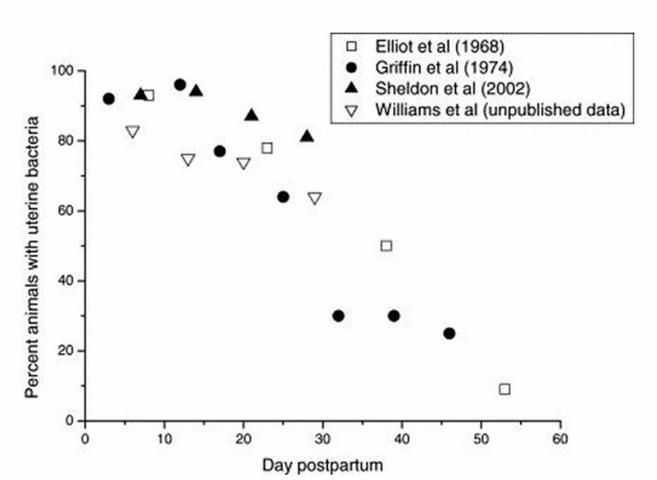
Credit: Sheldon and Dobson (2004)
Bacterial culture of the postpartum uterus yields a wide range of isolates (Elliot et al. 1968; Griffin et al. 1974; Sheldon et al. 2002; Galvão et al. 2009). A complete list of isolates can be found in Williams et al. (2005) (see Table 1). Mainly, Streptococcus spp., Staphylococcus spp., and Bacillus spp. were isolated from healthy cows in the first 10 days in milk (DIM), while Arcanobacterium pyogenes (A. pyogenes), Escherichia coli (E. coli), Fusobacterium necrophorum (F. necrophorum), and Prevotella melaninogenicus (P. melaninogenicus) were primarily isolated from cows with metritis (Bonnett et al. 1991; Bondurant 1999; Huszenicza et al. 1999; Gilbert et al. 2007).
A recent study that used metagenomic analysis to characterize the uterine flora in healthy and metritic cows observed that most clone sequences from the metritic cows were from the phylum Fusobacteria (58%–77%), which included F. necrophorum and F. necrophorum funduliforme, and Bacteroidetes (9%–16%), which included Porphyromonas and Bacteroides spp. Interestingly, P. melaninogenicus was not identified in the phylum Bacteroidetes and neither was A. pyogenes (from the phyla Actinobacteria) or E. coli (from the phyla Proteobacteria). In healthy cows, the clones were mainly from the phylum Proteobacteria, classis Gammaproteobacteria (100% in one farm and 42% in another), and the phylum Tenericutes (46% in one farm). The classis Gammaproteobacteria included Mannheimia varigena and Pasteurella hemolytica, and the phylum Tenericutes included Ureaplasma and Mycoplasma. Using regular culturing methods, the following have been cultured in cows with metritis: A. pyogenes (from 33% to 83%), E. coli (from 67% to 85%), and Gram negative anaerobes (F. necrophorum and Bacteroides spp.) (from 49% to 67%) (Bonnett et al. 1991; Huszenicza et al. 1999; Dohmen et al. 2000; Mateus et al. 2002; Földi et al. 2006). Nonetheless, E. coli is believed to give way to A. pyogenes later in lactation in cows with endometritis or pyometra (Olson et al. 1984; Gilbert et al. 2007).
These four main bacteria—A. pyogenes, E. coli, F. necrophorum, and P. melaninogenicus—are believed to work synergistically to cause uterine disease (Griffin et al. 1974; Ruder et al. 1981; Bonnett et al. 1991). In fact, E. coli might increase the susceptibility of the endometrium to subsequent infection with A. pyogenes (Olson et al. 1984; Gilbert et al. 2007; Williams et al. 2007), while A. pyogenes acts synergistically with F. necrophorum and P. melaninogenicus to enhance the severity of uterine disease (Griffin et al. 1974; Ruder et al. 1981; Bonnett et al. 1991). Among their effects, E. coli releases bacterial-wall lipopolysaccharides (LPS) (Williams et al. 2008); A. pyogenes produces the cholesterol-dependent cytotoxin pyolysin (Miller et al. 2007) and a growth factor for F. necrophorum (Sheldon and Dobson 2004); F. necrophorum produces a leukotoxin; and P. melaninogenicus produces a substance that inhibits phagocytosis (Sheldon and Dobson 2004).
E. coli and A. pyogenes have been more extensively studied than the other bacteria. Recently, it was observed that a specific E. coli causes uterine disease, which is different from known diarrhoeic or extra-intestinal pathogenic E. coli (Sheldon et al. 2010). This specific E. coli was named endometrial pathogenic E. coli or EnPEC. EnPEC was found to be more adherent and invasive to endometrial cells and also to stimulate greater production of prostaglandin E2 and interleukin 8 (Sheldon et al. 2010). Interleukin 8 is the main neutrophil chemokine. In another study, six virulence factors were found to be associated with metritis and endometritis: fimbriae components (fim) fimH, hemolyn A (hlyA), cytolethal distending toxin (cdt), group II capsule (kpsMII), invasion of brain endothelium (ibeA), and arginine succinyltransferase (astA). However, fimH was the most prevalent and significant factor (Bicalho et al. 2010). The authors concluded that E. coli carrying fimH and at least one of the other factors were pathogenic to dairy cows.
Arcanobacterium pyogenes has been highlighted in several studies as the main causative agent of endometrial damage and infertility (Ruder et al. 1991; Bonnett et al. 1991; Dohmen et al. 2000; Bondurant 1999). A recent study also tried to find specific virulent factors associated with uterine disease (Silva et al. 2008). They evaluated a series of virulence factors including pyolysin (plo), neuraminidases (nan) nanP, nanH, collagen-binding protein A (cbpA), fimA, fimC, fimE, and fimG, but were unable to find any association with incidence of metritis. They concluded that synergism between A. pyogenes and other bacteria and differential gene expression of virulence factors might be more important for establishment of infection. In another study, only fimA was found to be overrepresented in cows with metritis, while the other virulence factors were similarly found in both healthy and metritic cows (Santos et al. 2010).
Besides the four main bacteria involved in the pathogenesis of uterine disease, only one virus, bovine herpesvirus IV (BoHV-4), has been linked to uterine disease (Donofrio et al. 2007; Donofrio et al. 2008; Donofrio et al. 2009; Donofrio et al. 2010). Donofrio et al. (2007) observed that BoHV-4 had a tropism for endometrial cells and rapidly infected, replicated, and killed endometrial cells. BoHV-4 is usually isolated concurrently with bacteria that cause disease (Frazier et al. 2001; Monge et al. 2006); however, BoHV-4 itself can also stimulate an immune response by stimulating interleukin-8 production by endometrial cells. Interestingly, viral replication was stimulated by E. coli or its LPS (Donofrio et al. 2008), indicating synergism between E. coli infection and BoHV-4 replication.
In summary, pathogenic bacteria associated with metritis and endometritis are E. coli, A. pyogenes, F. necrophorum, and P. maleninogenicus. E. coli increases the susceptibility of the endometrium to subsequent infection with A. pyogenes, and A. pyogenes acts synergistically with F. necrophorum and P. melaninogenicus to enhance the severity of uterine disease. Among their effects, E. coli releases bacterial-wall LPS; A. pyogenes produces the cholesterol-dependent cytotoxin pyolysin (Miller et al. 2007) and a growth factor for F. necrophorum; F. necrophorum produces a leukotoxin; and P. melaninogenicus produces a substance that inhibits phagocytosis. A specific E. coli, called EnPEC, causes uterine disease, and the virulence factor fimH was mostly associated with disease. For A. pyogenes, fimA was the only virulence factor associated with uterine disease. BoHV-4 seems to be involved in the pathogenesis of uterine disease.
References
Bicalho, R.C., V.S. Machado, M.L. Bicalho, R.O. Gilbert, A.G. Teixeira, L.S. Caixeta, and R.V. Pereira. 2010. "Molecular and Epidemiological Characterization of Bovine Intrauterine Escherichia Coli." J. Dairy Sci. 93: 5818–30.
Bondurant, R.H. 1999. "Inflammation in the Bovine Female Reproductive Tract." J Anim Sci. 77 (Suppl 2): 101–10.
Bonnett, B.N., S.W. Martin, V.P. Gannon, R.B. Miller, and W.G. Etherington. 1991. "Endometrial Biopsy in Holstein-Friesian Dairy Cows III. Bacterial Analysis and Correlations with Histological Findings." Can. J. Vet. Res. 55: 168–73.
Dohmen, M. J., K. Joop, A. Sturk, P. E. Bols, and A. Lohuis. 2000. "Relationship between Intra-uterine Bacterial Contamination, Endotoxin Levels and the Development of Endometritis in Postpartum Cows with Dystocia or Retained Placenta." Theriogenology 54: 1019–32.
Donofrio, G., S. Herath, C. Sartori, S. Cavirani, C.F. Flammini, and I.M. Sheldon. 2007. "Bovine Herpesvirus 4 Is Tropic for Bovine Endometrial Cells and Modulates Endocrine Function." Reproduction 134: 183–97.
Donofrio, G., L. Ravanetti, S. Cavirani, S. Herath, A. Capocefalo, and I.M. Sheldon. 2008. "Bacterial Infection of Endometrial Stromal Cells Influences Bovine Herpesvirus 4 Immediate Early Gene Activation: A New Insight into Bacterial and Viral Interaction for Uterine Disease." Reproduction 136: 361–6.
Donofrio, G., V. Franceschi, A. Capocefalo, S. Cavirani, and I.M. Sheldon. 2009. "Isolation and Characterization of Bovine Herpesvirus 4 (BoHV-4) from a Cow Affected by Post-partum Metritis and Cloning of the Genome as a Bacterial Artificial Chromosome." Reprod. Biol. Endocrinol. 19: 83.
Donofrio, G., A. Capocefalo, V. Franceschi, S. Price, S. Cavirani, and I.M. Sheldon. 2010. "The Chemokine IL8 Is Up-regulated in Bovine Endometrial Stromal Cells by the BoHV-4 IE2 Gene Product, ORF50/Rta: A Step Ahead toward a Mechanism for BoHV-4 Induced Endometritis." Biol. Reprod. 83: 919–28.
Elliot, L., K.J. McMahon, H.T. Gier, and G.B. Marion. 1968. "Uterus of the Cow after Parturition: Bacterial Content." Am. J. Vet. Res. 29: 77–81.
Farin, P.W., L. Ball, J.D. Olson, R.G. Mortimer, R.L. Jones, W.S. Adney, and A.E. McChesney. 1989. "Effect of Actinomyces Pyogenes and Gram-negative Anaerobic Bacteria on the Development of Bovine Pyometra." Theriogenology 31: 979–89.
Földi, J., M. Kulcsár, A. Pécsi, B. Huyghe, C. de Sa, J.A. Lohuis, P. Cox, and G. Huszenicza. 2006. "Bacterial Complications of Postpartum Uterine Involution in Cattle." Anim. Reprod. Sci. 96: 265–81.
Frazier, K., M. Pence, M.J. Mauel, A. Liggett, M.E. Hines II, L. Sangster, H.D. Lehmkuhl, D. Miller, E. Styer, J. West, and C.A. Baldwin. 2001. "Endometritis in Postparturient Cattle Associated with Bovine Herpesvirus-4 Infection: 15 Cases." J. Vet. Diagn. Invest. 13: 502–8.
Galvão, K.N., L.F. Greco, J.M. Vilela, M.F. Sá Filho, and J.E. Santos. 2009. "Effect of Intrauterine Infusion of Ceftiofur on Uterine Health and Fertility in Dairy Cows." J. Dairy Sci. 92: 1532–42.
Gilbert, R.O., N.R. Santos, K.N. Galvão, S.B. Brittin, and H.B. Roman. 2007. "The Relationship between Postpartum Uterine Bacterial Infection (BI) and Subclinical Endometritis (SE)." J. Dairy Sci. 90(Suppl. 1): 469 (Abstr).
Griffin, J.F.T., P.J. Hartigan, and W.R. Nunn. 1974. "Non-specific Uterine Infection and Bovine Fertility I. Infection Patterns and Endometritis during the First Seven Weeks Post-partum." Theriogenology 1: 91-106.
Huszenicza, G., M. Fodor, M. Gacs, M. Kulscar, M.J. Dohmen, M. Vamos, L. Portokolab, T. Kegl, J. Bartyik, J.C. Janosi, and G. Szita. 1999. "Uterine Bacteriology, Resumption of Ovarian Activity and Fertility in Postpartum Cows Kept in Large-scale Dairy Herds." Reprod. Domest. Anim. 34: 237–45.
Mateus, L., L.L. da Costa, F. Bernardo, and J.R. Silva. 2002. "Influence of Puerperal Uterine Infection on Uterine Involution and Postpartum Ovarian Activity in Dairy Cows." Reprod. Domest. Anim. 37: 31–5.
Miller, A.N., E.J. Williams, K. Sibley, S. Herath, E.A. Lane, J. Fishwick, D.M. Nash, A.N. Rycroft, H. Dobson, C.E. Bryant, and I.M. Sheldon. 2007. "The Effects of Arcanobacterium pyogenes on Endometrial Function in Vitro, and on Uterine and Ovarian Function in Vivo." Theriogenology 68: 972–80.
Monge, A., L. Elvira, J.V. Gonzalez, S. Astiz, and G.J. Wellenberg. 2006. "Bovine Herpesvirus 4-associated Postpartum Metritis in a Spanish Dairy Herd." Res. Vet. Sci. 80:120–5.
Olson, J.D., L. Ball, R.G. Mortimer, P.W. Farin, W.S. Adney, and E.M. Huffman. 1984. "Aspects of Bacteriology and Endocrinology of Cows with Pyometra and Retained Fetal Membranes." Am. J. Vet. Res. 45: 2251–5.
Ruder, C.A., R.G. Sasser, R.J. Williams, J.K. Ely, R.C. Bull, and J.E. Butler. 1981. "Uterine Infections in the Postpartum Cow. II. Possible Synergistic Effect of Fusobacterium necrophorum and Corynebacterium pyogenes." Theriogenology 15: 573–580.
Santos, T.M., L.S. Caixeta, V.S. Machado, A.K. Rauf, R.O. Gilbert, and R.C. Bicalho. 2010. "Antimicrobial Resistance and Presence of Virulence Factor Genes in Arcanobacterium pyogenes Isolated from the Uterus of Postpartum Dairy Cows." Vet. Microbiol. 145: 84–9.
Santos, T.M., R.O. Gilbert, and R.C. Bicalho. 2011. "Metagenomic Analysis of the Uterine Bacterial Microbiota in Healthy and Metritic Postpartum Dairy Cows." J. Dairy Sci. 94: 291–302.
Sheldon, I.M., D.E. Noakes, A.N. Rycroft, D.U. Pfeiffer, and H. Dobson. 2002. "Influence of Uterine Bacterial Contamination after Parturition on Ovarian Dominant Follicle Selection and Follicle Growth and Function in Cattle." Reproduction. 123: 837–45.
Sheldon, I.M., and H. Dobson. 2004. "Postpartum Uterine Health in Cattle." Anim. Reprod. Sci. 82-83: 295–306.
Sheldon, I.M., A.N. Rycroft, B. Dogan, M. Craven, J.J. Bromfield, A. Chandler, M.H. Roberts, S.B. Price, R.O. Gilbert, and K.W. Simpson. 2010. "Specific Strains of Escherichia Coli Are Pathogenic for the Endometrium of Cattle and Cause Pelvic Inflammatory Disease in Cattle and Mice." PLoS One 5(2): e9192.
Silva, E., M. Gaivão, S. Leitão, B.H. Jost, C. Carneiro, C.L. Vilela, L. Lopes da Costa, and L. Mateus. 2008. "Genomic Characterization of Arcanobacterium pyogenes Isolates Recovered from the Uterus of Dairy Cows with Normal Puerperium or Clinical Metritis." Vet Microbiol. 132: 111–8.
Williams, E.J., D.P. Fischer, D.U. Pfeiffer, G.C. England, D.E. Noakes, H. Dobson, and I.M. Sheldon. 2005. "Clinical Evaluation of Postpartum Vaginal Mucus Reflects Uterine Bacterial Infection and the Immune Response in Cattle." Theriogenology. 63: 102–17.
Williams, E.J., D.P. Fischer, D.E. Noakes, G.C. England, A. Rycroft, H. Dobson, and I.M. Sheldon. 2007. "The Relationship between Uterine Pathogen Growth Density and Ovarian Function in the Postpartum Dairy Cow." Theriogenology 68: 549–59.
Williams, E.J., K. Sibley, A.N. Miller, E.A. Lane, J. Fishwick, D.M. Nash, S. Herath, G.C. England, H. Dobson, and I.M. Sheldon. 2008. "The Effect of Escherichia Coli Lipopolysaccharide and Tumour Necrosis Factor Alpha on Ovarian Function." Am. J. Reprod. Immunol. 60: 462–73.