What is bovine leukemia virus?
The bovine leukemia virus (BLV) is a pathogen that infects cattle lymphocytes (a type of white blood cell) in general by inserting its genomic material into the host genome, leading to lifelong infection (Ott et al. 2003). It has a similar genetic structure to human T-lymphotropic virus types I and II (HTLV-I and HTLV-II), which are known to cause cancers, such as adult T-cell leukemia/lymphoma in humans. Therefore, BLV can represent a model for investigation of the cause and development of leukemia in humans (Sagata et al. 1985). Bovine leukemia virus is the etiological agent of enzootic bovine leukosis (EBL), and the BLV belongs to the genus Deltaretrovirus, in the Retroviridae family. The first descriptions of EBL were made about 150 years ago in Germany, and reports of this disease became common after the Second World War. Enzootic bovine leukosis is a contagious chronic lymphoproliferative disorder of cattle, characterized by persistent lymphocytosis (a continuous increase of white blood cells) that can end with B-cell lymphoma, also known as Hodgkin lymphoma or non-Hodgkin lymphoma, especially in immune-compromised animals (Schwartz and Lévy 1994). In this case, a type of blood cell (B-cells) becomes abnormal and can no longer fight off the infection. Enzootic bovine leukosis is widely distributed. It was listed by the World Organisation for Animal Health (OIE) as a disease that could significantly impact international trade (Notsu et al. 2020).
Bovine leukemia virus infects cattle. It can present in one of three forms: 1) asymptomatic or aleukemic (cows do not display outward signs of disease); 2) persistent lymphocytosis (cows continuously show an increase in lymphocyte counts); and 3) leukemia or lymphoma/lymphosarcoma (leukemia is a type of tumor located in the bone marrow and blood, whereas lymphoma [or lymphosarcoma] is a tumor located in the lymph nodes). Approximately 60% to 65% of infected cattle are aleukemic, 30% to 40% have persistent lymphocytosis, and less than 5% develop malignant lymphosarcoma, which is considered the most common neoplastic disease identified in slaughtered cattle in the United States (Schwartz and Lévy 1994; Rodríguez et al. 2011; Nagy 2016). Older multiparous cows (≥5 parities) are most likely to display clinical signs, while cows under 2 years of age present most frequently as asymptomatic (Nagy 2016; Selim et al. 2020).
Prevalence of BLV
In the United States, the latest USDA survey showed that up to 83.9% of dairy operations were infected with BLV by bulk milk tank evaluation (herd prevalence is when at least one animal is BLV-positive within the herd), and at least one of five dairy cows tested positive for BLV serum antibodies (USDA-NAHMS 2008). A similar herd prevalence of BLV (89% BLV-positive herds) was shown previously in 1996 (USDA-NAHMS 1999). In the NAHMs study, when beef cattle were evaluated, approximately 38% of beef herds tested positive for BLV, while 10.3% of all beef cows had serum antibodies to BLV (USDA-NAHMS 1999). In this case, cow prevalence within a herd means the proportion of cows within the herd population with a BLV-positive status. In a recent survey performed from 2014 to 2015, BLV prevalence at the cow level was determined among cattle presented for slaughter in the US (Figure 1 and Tables 1–2) (Bauermann et al. 2017). Samples were sourced from five slaughter plants in the US (Figure 1; state location shown with an asterisk) and from most of the states. Of the total samples collected, 38.6% were positive for BLV (Table 1).
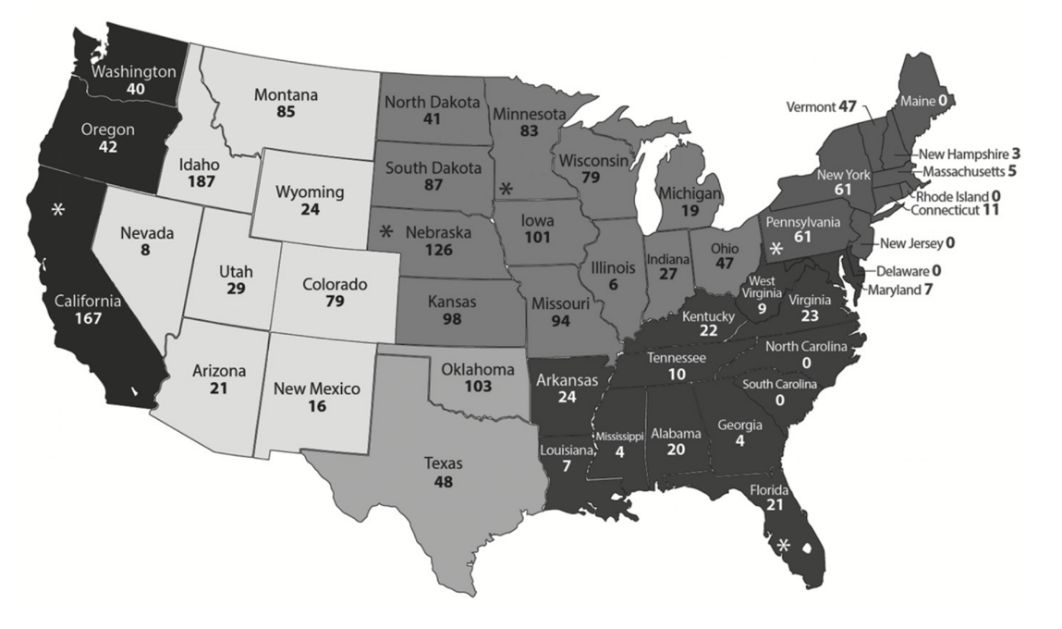
Credit: Bauermann et al. (2017)
The Southeast and Northeast regions of the US had the highest BLV prevalence with 45% and 54.3%, respectively (Table 1). When evaluating BLV serum prevalence by slaughter plant type (predominantly dairy, beef, or mixed), 47.7% of samples sourced from dairy slaughter plants tested positive for BLV compared to 33.6% of the beef cattle slaughter plants (Table 2) (Bauermann et al. 2017).
Table 1. Number and percentage of samples from slaughter plants that tested positive for BLV serum antibody relative to United States regions, from 2014 to 2015 (Bauermann et al. 2017).
In Canada, an updated epidemiologic survey suggests that the herd-based prevalence of BLV has increased up to 89% in dairy cattle. Individual-based BLV prevalence was estimated at 20.8% (VanLeeuwen et al. 2006). In addition, a recent Japanese study that used cattle movement network analysis showed that cattle movement is the primary method of inter-farm transmission of BLV, where approximately one in six introduced cattle will be infected with BLV. Therefore, the farms with a high number of cattle being introduced are the farms with an increased risk of BLV infection (Notsu et al. 2020).
Table 2. Number and percentage of samples from slaughter plants that tested positive for BLV serum antibody by slaughter plant type in the United States, from 2014 to 2015 (Bauermann et al. 2017).
Economic Importance of BLV
In both the dairy and beef industries, BLV infection results in high economic losses due to reduced milk quantity and quality (Da et al. 1993), lower fertility rates, increased heifer replacement costs, and veterinarian costs (consulting, medical care of the sick animals, and diagnostics). In addition, herds infected with BLV become unable to export cattle, semen, and embryos to countries that maintain BLV control programs, such as those in the European Union (Ferrer et al. 1979; Thurmond et al. 1985; Notsu et al. 2020). The economic losses of lymphosarcoma were estimated to be $412 per case, and the yearly direct losses in the dairy industry associated with clinical BLV infections were more than $500 million (Rhodes et al. 2003). The average annual cost in a 50% prevalence herd was nearly $6,400 per 100 milking cows.
The USDA National Animal Health Monitoring System (USDA-NAHMS) conducted two survey studies on US dairies in 1996 and 2007 and showed a high prevalence of BLV in dairy herds (83% of operations overall had bulk-tank tested positive for BLV in the USDA-NAHMS 2007 survey). Furthermore, 7.5% of dairy operations that submitted test samples for BLV had a positive laboratory BLV test in the previous year (USDA-NAHMS survey of 2007). Another study using data from these two NAHMS surveys estimated that herds with BLV-positive cows produced 218 kg less milk per cow annually than herds that were BLV-negative. Herds were determined to be BLV-negative based on the absence of serum antibodies to BLV in the agar gel immunodiffusion test. Only 114 herds out of 976 fit this criterion (Ott et al. 2003). The mean annual value of production per cow decreased by $1.28 for each 1% increase in herd BLV prevalence at a milk price of $0.29 per kg. Cows with persistent lymphocytosis showed progressive milk loss (Da et al. 1993). Intriguingly, BLV-positive cows showed significantly less milk production only in the early and middle stages of lactation than BLV-negative cows (Yang et al. 2016). Additionally, a higher somatic cell score in BLV-positive cows was also reported during the early and middle stages of lactation (Yang et al. 2016). It has been reported that the cattle infected with BLV showed an impaired immune response to J5 Escherichia coli bacterin and foot-and-mouth disease vaccines (Erskine et al. 2011; Puentes et al. 2016).
Transmission and Clinical Signs
Typically, BLV is not free in the peripheral blood. The virus is integrated with the lymphocytes of different body fluids, mostly milk and blood (Selim et al. 2020). Therefore, BLV is primarily transmitted through the blood of infected cattle, but to a lesser extent, it may transmit through milk, saliva, and semen (Benitez et al. 2019). Thus, the management practices that lead to direct exposure to infected blood, such as the use of common needles, blood-contaminated syringes, rectal sleeves, tattooing, and ear tagging, may increase the transmission of BLV infection. It is believed that BLV-infected cattle with persistent lymphocytosis can harbor more virus per lymphocyte in circulating blood and more readily transmit BLV (Juliarena et al. 2007).
It has been suggested that calves can become infected by mothers through infected milk. This route of infection has only been proven to occur in experimental settings despite BLV proviral DNA being present in the milk and colostrum of the infected dam (Ferrer and Piper1981). Calves may avoid contracting BLV by the passage of antibodies from the infected mother to the calf via her colostrum (Ruiz et al. 2018). Note that perinatal or postnatal transmission frequently occurs in infected herds, where the in-utero transmission rate is 4.8% (Lassauzet et al. 1991). Transmission is more likely to occur when the peripheral blood lymphocyte count during pregnancy is greater than 12,000 cells/µL, and in cows that developed malignant lymphoma (Lassauzet et al. 1991). It is currently unclear if insects play a role in BLV transmission from cow to cow. However, it has been found that decreasing the density of hematophagous insects in high-density environments such as milking areas and barns may potentially decrease the spread of BLV (Rodríguez et al. 2011).
The incubation period is the time of exposure and infection of a pathogen to the first appearance of associated clinical signs. The BLV incubation period ranges from 5 to 10 years, with an average of 7 years (Tsutsui et al. 2015). The specific clinical signs include lymphocytosis (increased lymphocyte counts in the peripheral blood), lymphadenopathy (peripheral and/or internal lymph node enlargement [Figures 2–6]) with or without brisket edema, jugular vein distention, bloat, rear limb weakness or paralysis, exophthalmia (protruding eyeball), and melena (feces with the presence of digested blood). Other specific clinical signs that are associated with other general clinical signs are fever, loss of appetite, dyspnea (labored breathing), tachycardia (increased heart rate), decreased milk production, and weight loss (Da et al. 1993; Nagy 2016; Yang et al. 2016).
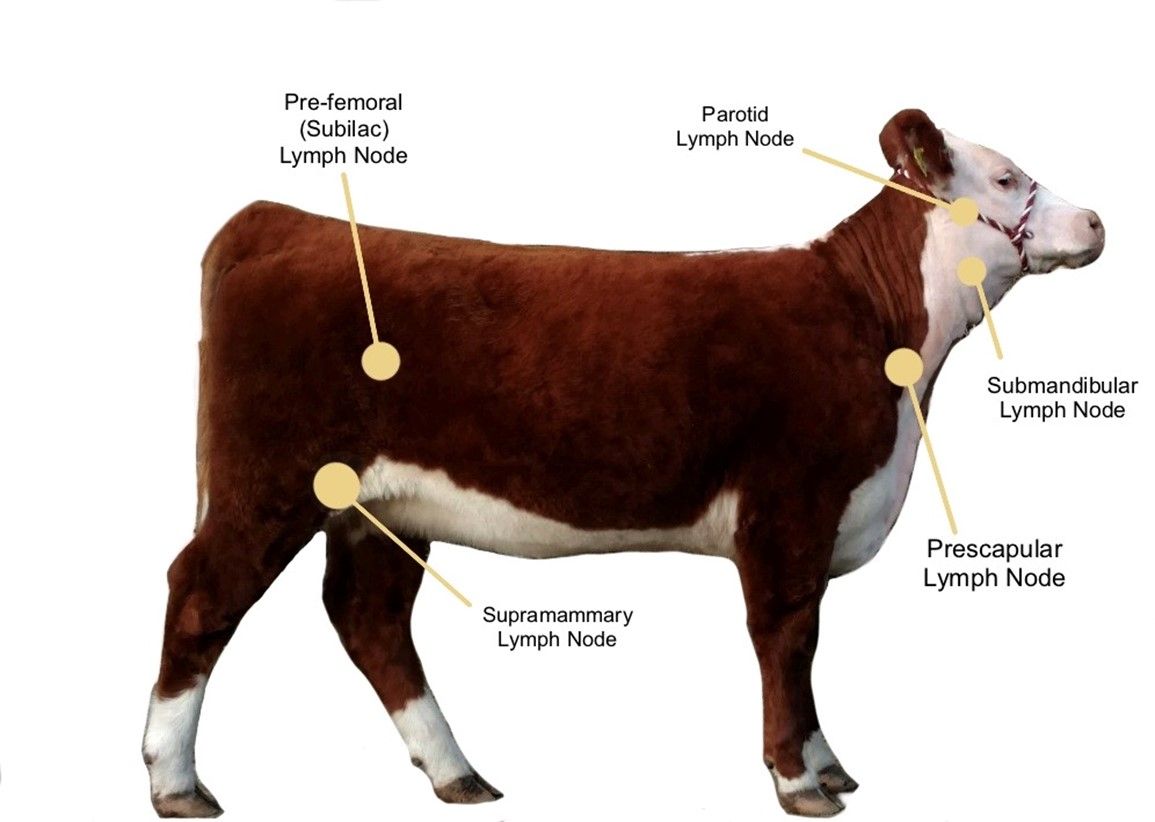
Credit: Kaitlyn Renegar, personal archive
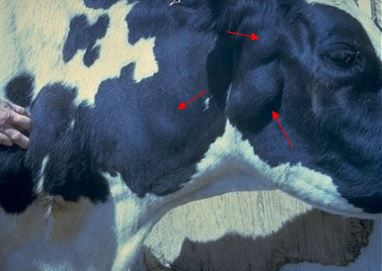
Credit: VeeproHolland (https://veepro.nl/animal-health/bovine-leukosis-bovine-lymphosarcoma-leukemia-malignant-lymphoma/)
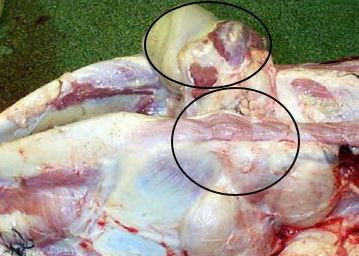
Credit: Scott (2011)
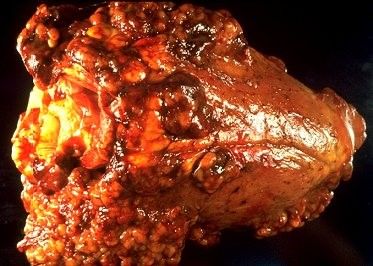
Credit: VeeproHolland (https://veepro.nl/animal-health/bovine-leukosis-bovine-lymphosarcoma-leukemia-malignant-lymphoma/)
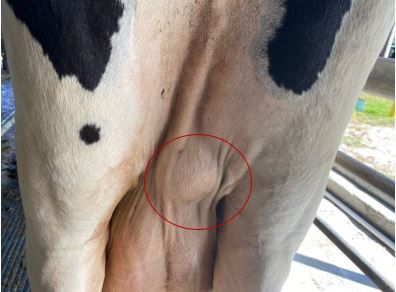
Credit: Kaitlyn Renegar, personal archive
Diagnosis
Typically, diagnosing the BLV infection includes direct detection of the virus in body fluids and/or indirect detection of serum BLV antibodies, also known as serology testing. A serology test looks for antibodies in the blood that fight the virus. Direct detection of the BLV antigen is performed through one of two methods: 1) virus isolation and propagation, which is not routinely used due to low viral gene expression and difficulties of virus propagation; or 2) direct detection of BLV virus using polymerase chain reaction (PCR), also used with limited success because of its highly mutable RNA genome. Additionally, traditional PCR is not designed to detect BLV in milk, which is the source of many transgenic proteins. However, real-time and nested PCR can provide a valuable method for the early detection of BLV and the confirmation of BLV infection from blood and milk samples (Kuckleburg et al. 2003; Rodríguez et al. 2011). The virus can also be detected by performing a PCR for proviral DNA detection specific to BLV. This type of test looks for the virus's genetic material, which is more efficient. PCR is the better diagnostic test for BLV, but it costs more.
Commercial ELISA kits are the most popular serological test for detecting BLV infection in cattle because they are rapid, inexpensive, and easy to interpret. However, the antibodies against BLV may not be produced until 14 weeks after infection, when the infected animals are viremic and transmit the BLV to other animals. Additionally, the serological tests cannot differentiate between active (i.e., vaccination) and passive immunity (i.e., immunoglobulins transfer via colostrum) (Kuckleburg et al. 2003; Rodríguez et al. 2011). For instance, bulk-tank milk ELISA is frequently applied to surveillance of BLV, where the infected animal remains infected for life and generates a continuous antibody response which adds credibility to serological tests (Nekouei et al. 2016).
Treatment
Currently, there are no treatments available for animals infected with BLV. Parenteral corticosteroids, such as dexamethasone, might help to decrease the severity of disease in cattle as suggested in the Merck Veterinary Manual (Nagy 2016). However, the lack of scientific evidence justifying the benefit of corticosteroid use needs to be considered. In addition, there is a research study that suggests corticosteroids can reduce some types of lymphocyte cells, and this reduction is associated with rapid BLV disease progression (Bloom et al. 1979). Therefore, caution should be exercised when recommending and using corticosteroids in cattle. Prevention and control of infection are the only effective methods to stop the spread of BLV between animals.
Prevention and Control
To date, there is no available vaccine against BLV in cattle, and the only way to prevent the spread of BLV among cattle is to control the transmission between infected animals and susceptible cattle. Control of BLV infection at the national level is usually performed through one or more of the following three approaches: 1) test and segregation; 2) test and slaughter; and 3) test and manage, and apply effective biosafety and management measures to minimize or prevent BLV exposure (Table 3). The selection and the success of each strategy are mainly dependent on having a reliable estimate of the within-herd prevalence (Rodríguez et al. 2011). Currently, in the United States, the test-and-removal strategy is voluntary.
Table 3. Approaches for control and prevention of BLV with advantages and disadvantages (Rodríguez et al. 2011).
Note that the first and second approaches will not eradicate the BLV from the herd without applying restricted hygienic management procedures to reduce exposure to BLV. Such management procedures include avoiding the sharing of needles, changing rectal sleeves/gloves between each palpation, and thoroughly disinfecting tools and equipment that can share blood (tattooing pliers, ear tag pliers, IV tubing, dehorning instruments, castration knives, hoof trimming knives, hormone implant devices, and similar instruments), as well as using insecticide to lower the blood-feeding insects' population (Rodríguez et al. 2011). A boundary of at least 200 meters between the primary herd and the BLV-infected herd has been suggested to prevent transmission (Shettigara et al. 1989).
In the 1960s, Europe took action to prevent the spread of BLV, and has since eradicated it from 22 countries (EFSA AHAW Panel 2015). The current recommended steps to eliminate BLV from the herd are: 1) identify infected animals using commercially available serologic tests; 2) cull seropositive animals immediately; 3) retest the herd in 30–60 days; 4) use PCR to test young calves and as a complementary test to clarify test results in herds with low prevalence of BLV infection; and 5) repeat testing and cull until entire herd tests negative (Nagy 2016). The test must be repeated every six months for two years with no positive tests until the herd can be declared BLV-free. Any new animal that is introduced to the herd must have two negative tests 30 and 60 days before arriving at the farm. Importantly, two consecutive negative tests are required before a new animal enters the herd due to the latency period, which is the time between infection and the development of antibodies (Nagy 2016).
Final Remarks
Bovine leukosis is a critically important disease, currently without effective treatment for clinically affected cattle. With dedication and an aggressive approach, most European countries, New Zealand, and Australia have eradicated the virus, further showing that eradication is possible if the appropriate measures are taken. BLV eradication within the US cattle industry has had limited success. A few possible reasons the US has made little progress in eliminating BLV include but are not limited to the economic costs involved, management limitations, lack of effective vaccination, and large operation sizes. US cattle herds are still susceptible to BLV infection, and the associated losses have a negative impact on cattle production and performance. These effects are even more pronounced when the lymphoma-leukemia form is present in the herd due to carcass condemnation at the slaughterhouse in cattle with clinical signs. Note that most research related to the determination of seroprevalence of BLV in the US was conducted on dairy cattle. Similar seroprevalence studies should be conducted on beef cattle herds. Education and research aimed at BLV control and prevention strategies are of value and warranted for the US cattle industry.
If you suspect cases of cattle with bovine leukemia virus in your herd, contact your local or state veterinarian to get help with diagnosis and future management practices to reduce further losses related to this disease.
References
Bauermann, F. V., J. F. Ridpath, and D. A. Dargatz. 2017. “Bovine Leukemia Virus Seroprevalence among Cattle Presented for Slaughter in the United States.” Journal of Veterinary Diagnostic Investigation 29 (5): 704–706. https://doi.org/10.1177/1040638717702183
Benitez, O. J., J. N. Roberts, B. Norby, P. C. Bartlett, and J. E. Maeroff. 2019. “Lack of Bovine Leukemia Virus Transmission during Natural Breeding of Cattle.” Theriogenology:187–190. https://doi.org/10.1016/j.theriogenology.2018.12.005
Bloom, J. C., S. J. Kenyon, and T. G. Gabuzda. 1979. “Glucocorticoid Effects on Peripheral Blood Lymphocytes in Cows Infected with Bovine Leukemia Virus.” Blood 53 (5): 899–912. https://doi.org/10.1182/blood.V53.5.899.899
Da, Y., R. D. Shanks, J. A. Stewart, and H. A. Lewin. 1993. “Milk and fat yields decline in bovine leukemia virus-infected Holstein cattle with persistent lymphocytosis.” Proc. Natl. Acad. Sci. USA. 90:6538–6541. https://doi.org/10.1073/pnas.90.14.6538
EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare). 2015. “Scientific Opinion on Enzootic Bovine Leukosis.” EFSA Journal 13 (7): 4188. https://doi.org/10.2903/j.efsa.2015.4188
Erskine, R. J., P. C. Bartlett, K. M. Sabo, and L. M. Sordillo. 2011. “Bovine Leukemia Virus Infection in Dairy Cattle: Effect on Serological Response to Immunization against J5 Escherichia coli Bacterin.” Veterinary Medicine International.:915747. https://doi.org/10.4061/2011/915747
Ferrer, J. F., R. R. Marshak, D. A. Abt, and S. J. Kenyon. 1979. “Relationship between Lymphosarcoma and Persistent Lymphocytosis in Cattle: A Review.” J. Am. Vet. Med. Assoc. 175 (7): 705–708.
Ferrer, J. F., and C. E. Piper. 1981. “Role of Colostrum and Milk in the Natural Transmission of the Bovine Leukemia Virus.” Cancer Research. 41 (12 Pt. 1): 4906–4909.
Juliarena, M. A., S. E. Gutierrez, and C. Ceriani. 2007. “Determination of Proviral Load in Bovine Leukemia Virus-Infected Cattle with and without Lymphocytosis.” Am. J. Vet. Res. 68 (11): 1220–1225. https://doi.org/10.2460/ajvr.68.11.1220
Kuckleburg, C. J., C. C. Chase, E. A. Nelson, S. A. E. Marras, M. A. Dammen, and J. Christopher-Hennings. 2003. “Detection of Bovine Leukemia Virus in Blood and Milk by Nested and Real-Time Polymerase Chain Reactions.” J. Vet. Diagn. Invest. 15 (1): 72–76. https://doi.org/10.1177/104063870301500117
Lassauzet, M. L., M. C. Thurmond, W. O. Johnson, and C. A. Holmberg. 1991. “Factors Associated with In Utero or Periparturient Transmission of Bovine Leukemia Virus in Calves on a California Dairy.” Can. J. Vet. Res. 55 (3): 264–268.
Nagy, D. W. 2016. “Bovine Leukosis.” In The Merck Veterinary Manual, edited by S. Aiello and M. A. Moses. 743–745. Kenilworth: Merck & Co., Inc.
Nekouei, O., J. Durocher, and G. Keefe. 2016. “Diagnostic Performance of an Indirect Enzyme-Linked Immunosorbent Assay (ELISA) to Detect Bovine Leukemia Virus Antibodies in Bulk-Tank Milk Samples.” Can. Vet. J. 57 (7): 778–780.
Notsu, K., A. Wiratsudakul, S. Mitoma, H. El Daous, C. Kaneko, H. M. El-Khaiat, J. Norimine, and S. Sekiguchi. 2020. “Quantitative Risk Assessment for the Introduction of Bovine Leukemia Virus-Infected Cattle Using a Cattle Movement Network Analysis.” Pathogens. 9 (11): 903. https://doi.org/10.3390/pathogens9110903
Ott, S. J., R. Johnson, and S. J. Wells. 2003. “Association between Bovine-Leukosis Virus Seroprevalence and Herd-Level Productivity on US Dairy Farms.” Prev. Vet. Med. 61 (4): 249–262. https://doi.org/10.1016/j.prevetmed.2003.08.003
Puentes, R., L. De Brun, A. Algorta, V. Da Silva, F. Mansilla, G. Sacco, S. Llambí, and A. V. Capozzo. 2016. “Evaluation of Serological Response to Foot-and-Mouth Disease Vaccination in BLV-Infected Cows.” BMC Veterinary Research 12 (1): 119. https://doi.org/10.1186/s12917-016-0749-x
Rhodes, J. K., K. D. Pelzer, and Y. J. Johnson. 2003. “Economic Implications of Bovine Leukemia Virus Infection in Mid-Atlantic Dairy Herds.” J. Am. Vet. Med. Assoc. 223 (3): 346–352. https://doi.org/10.2460/javma.2003.223.346
Rodríguez, S. M., A. Florins, N. Gillet, A. de Brogniez, M. T. Sánchez-Alcaraz, M. Boxus, F. Boulanger, G. Gutiérrez, K. Trono, I. Alvarez, L. Vagnoni, and L. Willems. 2011. “Preventive and Therapeutic Strategies for Bovine Leukemia Virus: Lessons for HTLV.” Viruses 3 (7): 1210–1248. https://doi.org/10.3390/v3071210
Ruiz, V., N. G. Porta, M. Lomónaco, K. Trono, and I. Alvarez. 2018. “Bovine Leukemia Virus Infection in Neonatal Calves. Risk Factors and Control Measures.” Frontiers in Veterinary Science 5:267. https://doi.org/10.3389/fvets.2018.00267
Sagata, N., T. Yasunaga, J. Tsuzuku-Kawamura, K. Ohishi, Y. Ogawa, and Y. Ikawa. 1985. “Complete Nucleotide Sequence of the Genome of Bovine Leukemia Virus: Its Evolutionary Relationship to Other Retroviruses.” Proc. Natl. Acad. Sci. USA. 82 (3): 677–681. https://doi.org/10.1073/pnas.82.3.677
Schwartz, I., and D. Lévy. 1994. “Pathobiology of Bovine Leukemia Virus.” Veterinary Research 25 (6): 521–536.
Scott, P. 2011. “Lymphatic and Other Tumours in Cattle.” The National Animal Disease Information Service (NADIS). https://www.nadis.org.uk/disease-a-z/cattle/lymphatic-and-other-tumours-in-cattle/
Selim, A., A. A. Megahed, S. Kandeel, and A. Abdelhady. 2020. “Risk Factor Analysis of Bovine Leukemia Virus Infection in Dairy Cattle in Egypt.” Comp. Immunol. Microbiol. Infect. Dis. 72:101517. https://doi.org/10.1016/j.cimid.2020.101517
Shettigara, P. T., B. S. Samagh, and E. M. Lobinowich. 1989. “Control of Bovine Leukemia Virus Infection in Dairy Herds by Agar Gel Immunodiffusion Test and Segregation of Reactors.” Can. J. Vet. Res. 53 (1): 108–110.
Thurmond, M. C., G. R. Lapuz, T. B. Farver, and G. C. Mandac. 1985. “Retrospective Study of Four Years of Carcass Condemnation Rates for Malignant Lymphoma in California Cows.” Am. J. Vet. Res. 46 (6): 1387–1391.
Tsutsui, T., S. Kobayashi, Y. Hayama, and T. Yamamoto. 2015. “Fraction of Bovine Leukemia Virus-Infected Dairy Cattle Developing Enzootic Bovine Leukosis.” Prev. Vet. Med. 124:96–101. https://doi.org/10.1016/j.prevetmed.2015.11.019
USDA (Veterinary Services Centers for Epidemiology and Animal Health). 2008. “Info Sheet: Bovine Leukosis Virus (BLV) on U.S. Dairy Operations, 2007.” Animal and Plant Health Inspection Services, U.S. Department of Agriculture. #N526.0708. https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy07/Dairy07_is_BLV.pdf
USDA (Veterinary Services Centers for Epidemiology and Animal Health). 1999. “Info Sheet: Bovine Leukosis Virus (BLV) in U.S. Beef Cattle.” Animal and Plant Health Inspection Services, U.S. Department of Agriculture. #N526.0708. https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef97/Beef97_is_BLV.pdf
VanLeeuwen, J. A., A. Tiwari, J. C. Plaizier, and T. L. Whiting. 2006. “Seroprevalences of Antibodies against Bovine Leukemia Virus, Bovine Viral Diarrhea Virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in Beef and Dairy Cattle in Manitoba.” Can. Vet. J. 47 (8): 783–786.
Yang, Y., W. Fan, Y. Mao, Z. Yang, G. Lu, R. Zhang, H. Zhang, C. Szet, and C. Wang. 2016. “Bovine Leukemia Virus Infection in Cattle of China: Association with Reduced Milk Production and Increased Somatic Cell Score.” J. Dairy Sci. 99 (5): 3688–3697. https://doi.org/10.3168/jds.2015-10580