The intended audience for this publication is commercial poultry producers and poultry veterinarians. This publication aims to provide an update and summary of fowl adenovirus hepatitis.
The causative agent of inclusion body hepatitis (IBH) and hepatitis hydropericardium syndrome (HHS) is a fowl adenovirus. It is a nonenveloped virus. The virus contains 3 main structural proteins (Figure 1). The fiber knob attaches to the target cell receptor sites, thus determining tissue tropisms. The fiber knobs attach to the body of the virus at the penton bases. The penton base has toxic activity that causes immune system damage and leads to immune suppression. The hexon subunits are used to determine antigenic group, type, and subtype. These proteins surround the double-stranded DNA of the virus (Figure 2).
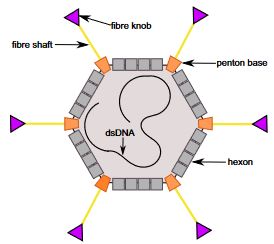
Credit: Aleksandra Cecylia Stasiak and Thilo Stehle
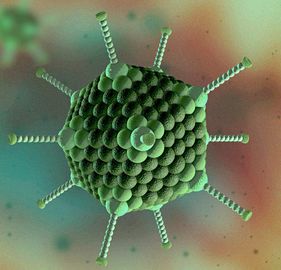
Credit: Springer Nature
Adenoviruses are ubiquitous in the poultry industry. Many adenovirus infections are subclinical or, in some cases, not associated with any disease. Adenoviruses are spread both vertically and horizontally from chickens. Currently, there are two forms of fowl adenovirus hepatitis in commercial poultry: inclusion body hepatitis (IBH) and hepatitis hydropericardium syndrome (HHS).
Inclusion body hepatitis (IBH) has been present in the poultry industry worldwide for many years. Infection was common based on serologic studies and often subclinical. Infection was spread globally, but did not cause significant losses, so little effort was made to eliminate it. It was recognized that clinical disease only occurred in poultry that were severely immunocompromised. For example, when immunosuppressive Gumboro disease first appeared in the early 1960s and caused widespread and severe immune suppression, losses from IBH also increased. However, mortality from IBH outbreaks normally were in the 1%–3% range. Clinical outbreaks of IBH continue to occur sporadically worldwide.
Gross liver lesions and increased mortality occurred in IBH-infected broilers that were markedly immune suppressed. These outbreaks have been associated with coinfection with immunosuppressive diseases such as infectious bursal disease (IBD), chicken anemia virus (CAV), or feed with high levels of mycotoxins. Mortality is usually in the 1%–3% range and involves serotypes 2, 8, and 11.
In 1987, a mutation of serotype 4 of IBH was reported in Pakistan. Due to the lesions present, it was named hepatitis hydropericardium syndrome (HHS). The disease spread to many countries, including Mexico, Dominican Republic, Turkey, Russia, India, China, Egypt, and Guyana, as well as other countries in South and Central America. Characteristic gross lesions included excess accumulation of fluid in the pericardial sac and necrotic foci in the liver. This mutation was characterized by very high morbidity and mortality in the 20%–70% range.
HHS differed from IBH in that it was a primary pathogen in poultry, not requiring immune suppression associated with IBD or CAV. The mutation occurred in the penton base protein which was able to cause significant primary immune suppression by destroying lymphocytes in the bursa of Fabricius, spleen, and thymus.
As HHS was much more severe, a better understanding of the behavior of this virus was needed to help in its control. It was shown that once a chicken was infected, it became a lifelong carrier. Infection of broiler breeders during the growing stage or while in production was subclinical but resulted in seroconversion. When all breeders became infected in a flock, virus shedding in the egg and by horizontal route decreased. Reactivation of the latent virus occurred with stress and virus shedding occurred again. In other words, an infected breeder flock is always a danger because it can potentially shed the virus again to infect other chickens both vertically and horizontally.
HHS Transmission in Broilers
If the virus is horizontally or egg-transmitted to broilers, the virus may remain latent until maternal antibodies wane by 3–4 weeks of age. If horizontally or egg-transmitted in the absence of maternal antibodies, the clinical disease in broilers can occur at 1–2 weeks of age. Most cases of HHS occur at 3–6 weeks of age, suggesting most broiler breeders are infected and seropositive.
HHS Clinical Findings in Broilers
Often, a sudden increase in mortality from 20%–70% is found. This depends on the challenge dose, presence of passive immunity, and stress on the broilers. Mortality usually starts at 3–4 weeks of age, reaches a peak at 4–5 weeks of age, and then subsides at 5–6 weeks of age. About 4–6 hours prior to death, chickens often become lethargic. There is an abrupt onset of mortality with lethargy, ruffled feathers, and yellow mucoid droppings.
HHS Gross Lesions in Broilers
Approximately 3–8 mL of a straw-colored clear fluid is found in the pericardial sac (Figure 3). This becomes gel-like upon exposure to air during the necropsy. The liver is enlarged and friable with multifocal areas of necrosis (Figure 4). Kidneys may be pale, swollen, and mottled in appearance. An accumulation of fluid is often found in the lungs. The carcass has a generalized congestion due to the febrile state. Skin and body fat may appear yellow in color due to liver damage. Petechial and ecchymotic hemorrhages may be present in the leg muscles.
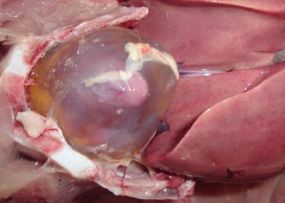
Credit: Mohamed Hossiny
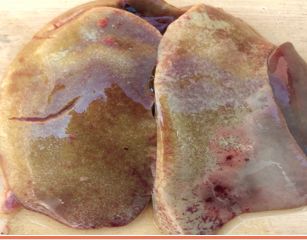
Credit: Mohamed Hossiny
HHS Histopathological Lesions in Broilers
Multifocal to extensive degeneration and necrosis occur in hepatocytes. Basophilic or eosinophilic intranuclear inclusion bodies may be present in the hepatocytes. The bursa of Fabricius may have reduced follicular size and lymphoid depletion. Glomerulonephritis with intraluminal inflammatory cells is found in the kidneys.
Diagnosis of HHS in Broilers
- Typical clinical presentation includes abrupt increase in broiler mortality at 3–6 weeks of age.
- ELISA serology is of limited value here because it cannot differentiate among serotypes. It is common to find adenovirus titers in healthy broilers due to infection with mild, ubiquitous strains.
- Histological detection of basophilic or eosinophilic intranuclear inclusion bodies in hepatocytes and extensive liver necrosis occurs.
- PCR can detect avian adenovirus DNA in the liver. However, nucleotide sequencing is needed to determine genotype because subclinical and nonpathogenic adenoviruses are ubiquitous.
Prevention and Control of HHS
If a broiler company is free of the infection, make sure to only use chicks or fertile eggs from breeders free of infection because the virus can be vertically transmitted. Proper cleaning and disinfection of the farm and equipment between cycles to reduce virus load are important. Adenoviruses are nonenveloped and therefore are very resistant in the environment. It is difficult to eliminate adenovirus from the premises, but reduction of challenge load is very beneficial. Biosecurity is critical to prevent horizontal transmission of this virus among farms. Reduce stressors on the farm. It has been demonstrated that broilers under stress have much more severe losses. Immune suppression results in increased losses, so take steps to prevent it. Ensure breeders have strong infectious bursal disease and chicken anemia virus vaccination programs so that broiler stock can receive high and uniform levels of maternal antibodies to prevent early immune suppression. Additionally, monitor mycotoxin levels in the feed because these toxins will also cause immune suppression.
Since 1987, when serotype 4 had undergone mutation to cause hepatitis hydropericardium syndrome, serotypes 2, 8, and 11 have also mutated and increased in virulence. Serotype 4 is still the most virulent form of HHS. Serotype 8 has been further classified as 8a and 8b. Strain 8a is very common. Strains 8a and 8b can cause significant clinical disease. If strains 8a and 8b infect broilers that are stressed and immune suppressed, very elevated mortality will occur and chickens will have hydropericardium, liver enlargement with extensive necrosis, and pale, swollen kidneys. Serotypes 2 and 11 are mostly subclinical.
In the commercial poultry industry, HHS severity can be highly variable, depending on the stress level of the flock. For example, in hot and humid climates, the disease is much more severe in open-sided houses compared to properly run tunnel-ventilated houses. The disease is much more severe in flocks that have feed with high mycotoxin levels, or that have damage to bursas of Fabricius and resulting severe immune suppression. In companies that have increased downtime between flocks from 1 week to 3 or 4 weeks, the losses from HHS decline substantially. Heat treatment of litter by windrowing has also been shown to significantly reduce viral load in a poultry house. The severity of losses from HHS is highly variable based on stress level in the flock and challenge dose.
Vaccination against HHS
Vaccination against egg drop syndrome, another adenovirus infection of poultry, provides no cross-protection against HHS. There is limited cross-protection among serotypes of the avian adenoviruses. Therefore, it is imperative to characterize the virus and use a vaccine containing an antigenically homologous virus for best protection. To achieve best control of HHS in your broiler flocks, injection of breeder stock with 2 killed adenovirus vaccines containing homologous serotypes is recommended. This program has successfully resulted in control of losses in broilers. In companies that do not have breeders and thus purchase fertile eggs or 1-day-of-age chicks where it is not possible to ensure breeder vaccination, vaccination of broilers is attempted. In some cases, a single injection is performed in the hatchery; in other cases, a field vaccination is also included. This has resulted in some reduction in losses, but not in satisfactory control. In the United States, fowl adenovirus infection is widespread, and as of this writing, no commercial vaccine has been approved. This has resulted in most companies contracting to have an autogenous vaccine made for breeder application. This is an expensive process that will continue until an approved commercial vaccine is available. The advantage of the autogenous vaccine is that it ensures the virus in the vaccine is homologous with the field virus.