Introduction
Tomato is the most important vegetable crop in Florida, which leads all other states in fresh market production. The sweetpotato whitefly, Bemisia tabaci (SWF), was reported in Florida as early as 1900 by Quaintance. However, it was not until the invasion of a new biotype or species in 1986, named successively "biotype B," Bemisia argentifolii, and more recently, Middle East-Asia Minor 1 (MEAM1), that it attained the status of key tomato pest.
The adult SWF is a small insect, approximately 1/16 of an inch in length and white in color due to a powdery covering of wax. Wings are held tent-like over the body while at rest in contrast to the somewhat larger greenhouse whitefly, which holds the wings flat. Whiteflies are usually found on the undersides of leaves, especially young leaves where the yellow, football-shaped eggs are laid upright and are attached by a tiny stalk inserted into the leaf surface. The mobile first instar, or crawler stage, hatches from the egg and settles on the leaf, where it develops through three immobile, semi-transparent, scale-like nymphal stages, the last of which is yellowish with red eyes. As the plant grows, leaves bearing successive nymphal stages tend to be found lower down on the plant. Development from egg to adult can be as short as two weeks during warm weather.

Credit: Lyle Buss, UF/IFAS
Whiteflies feed by sucking sap from the leaves, and in doing so can debilitate the plant, promote growth of sooty mold on excreted honeydew, cause irregular ripening of tomato, and transmit viral pathogens, the worst of which is Tomato yellow leaf curl virus (TYLCV). The virus can be acquired by the whitefly as a nymph or adult and is retained for the duration of life. TYLCV is common in Florida and causes severe stunting, shortened internodes, interveinal chlorosis, upward curled leaves, and flower abscission. Fruit set shuts down once a plant is infected, and early infection may result in little or no yield. Therefore, protecting young crops from whitefly attack is a priority.

Credit: UF/IFAS
Cultural Control
Whiteflies can be partially excluded from greenhouses with insect-proof screens or netting, but in Florida, most tomato production is open field. Therefore, the best way to protect new crops is to distance them in time and space from sources of whiteflies and virus. A tomato-free summer period of two months can provide an effective barrier against carryover of whiteflies and virus from the spring to fall crops in the southern production areas, whereas winter has typically provided a better break in the south-central area and north Florida/Georgia production areas. For this reason, whitefly populations and virus incidence are typically worse during spring in the south and during fall in the south-central region. Rapid crop destruction and field sanitation are important cultural management practices, and successive plantings in close proximity should be avoided.
Alternate hosts of TYLCV include common bean (Phaseolus vulgaris) and some varieties of pepper (Capsicum annuum), including varieties that do not show virus symptoms (Polston et al. 2006). Weeds have been identified as important reservoirs of TYLCV in some parts of the world (Papayiannis et al. 2011). However surveys of weeds in Florida have not revealed significant wild hosts of the virus (Polston et al. 2009).
Another form of cultural control is the use of TYLCV-tolerant cultivars that express little or no disease symptoms. The genetic sources of resistance are increasing in number, and the horticultural characteristics of virus-tolerant varieties are becoming more acceptable to growers and the marketplace.

Credit: UF/IFAS
Biological Control
Whiteflies have many natural enemies, including parasitic wasps, and predators, such as plant bugs, mites, ladybird beetles, lacewings and others. Pest management including whitefly control in much greenhouse tomato production of Europe, Canada, and to a lesser extent the United States, includes or depends completely upon biological control aided by TYLCV-tolerant cultivars. The predaceous mite Amblyseius swirskii is widely used to control whiteflies in non-tomato crops, while in tomato, the parasitic wasp Eretmocerus spp. and predaceous plant bugs, such as Nesidiocorus tenuis, are used alone or in combination to control whiteflies. Although these practices are not yet common in the open-field cropping systems of Florida, the biological control that occurs in weeds and other non-sprayed host plants during fallow periods is a significant factor in reducing whitefly populations during fallow periods.
Insecticidal Control—Soil Applications
Florida growers rely heavily on insecticidal programs to combat whiteflies and subsequent virus outbreaks. Young plants are most impacted by virus, so protection early in the cropping cycle is essential. Soil-applied systemic insecticides are translocated to growing tissues where whiteflies typically reside and are therefore necessary to provide the continuous protection needed by young, rapidly growing plants. Transplants in the production house are usually drenched with a systemic insecticide about a week before planting and again at or shortly after transplanting in the field. Transplant house applications have typically been made with imidacloprid and the field drench with imidacloprid, thiamethoxam, or dinotefuran, all neonicotinoid (group 4A) insecticides (Table 1).
Even though most Florida tomato growers refrain from making foliar applications of neonicotinoids, widespread dependence and overuse of these products has resulted in reduced effectiveness. The anthranilic diamide cyantraniliprole (also known as cyazypyr, group 28) was registered for use on Florida tomato in 2014 and is available for application as a soil formulation (Verimark™). It can be applied in the soil or transplant tray. Flupyradifurone (Sivanto Prime®) is a butenolide insecticide (4D) with the same mode of action as the neonicotinoids (4A) and should be grouped with the neonicotinoids for the purposes of resistance management. Confining use of each mode of action to a single soil application per crop will help preserve effectiveness over time. Later soil applications with a different mode of action can be made by injection through drip irrigation once roots systems have developed.
Foliar Applications
Careful scouting is important to determine when the effectiveness of the initial soil application has worn off. At this point, it is typically necessary to apply foliar sprays. Numerous insecticides are available for this purpose, some being more effective on nymphs and others on adults. Once the crop has established, and barring large scale migration of adults from offsite, the main objective will be controlling in-field reproduction, i.e. nymphs. Useful products for this include cyantraniliprole = cyazypyr (Exirel™), buprofezin (Courier™), pyriproxyfen (Knack®), spiromesifen (Oberon®), spirotetramat (Movento®), soaps and oils. These are listed by mode of action in Table 2 to assist in planning a program of rotation to avoid insecticide resistance. In addition, several microbial and botanical insecticides are available which can also be used. Another advantage to products mentioned above is their relative selectivity, and thus greater compatibility, with beneficial insects and mites compared to broad-spectrum insecticides that are typically used to target adults and are best used late in the crop if at all. Control of adults with products such as pyrethroids (group 3) with or without organophosphates (group 1B) may be needed at the end of the crop to limit migration to new crops, although 1B products are less compatible with harvesting due to longer pre-harvest intervals. Control of other pests such as leafminers, caterpillars or spider mites may also need to be considered and activity of insecticides on these is also mentioned in Table 2.
A resistance monitoring program for the neonicotinoids in southern Florida demonstrated that tolerance in B. tabaci increased eightfold on the average from 2000 to 2006 for imidacloprid and about fifteenfold from 2003 to 2006 for thiamethoxam. Ongoing resistance monitoring (2014 to the present) indicates that tolerance of sweetpotato whitefly to neonicotinoids varies among different populations in Florida, although in most areas susceptibility to dinotefuran remains high. All neonicotinoids can be applied as a spray to the foliage, and clothianidin (Belay®), dinotefuran (Venom®, Scorpion®), imidacloprid (Admire Pro® and others), thiamethoxam (Platinum®), and Sivanto are effective when applied to the soil. From the perspective of resistance management, the use of Group 4A insecticides for management of B. tabaci and TYLCV in tomato should be limited to nursery 1 week prior to planting, a single soil application, or foliar spray up to six weeks after planting but no later.
Verimark®, the soil-applied formulation of cyantraniliprole (= cyazypyr) is a systemic group 28 insecticide effective against SWF. Like the neonicotinoids, Verimark can be applied as a drench in transplant water or it may be injected through the drip once plants have established an adequate root system. If an at-plant application is chosen, then a neonicotinoid could be applied later to the foliage or preferably through the drip or as a drench. Again, in the interest of maintaining effectiveness of these valuable products, only one application each of an insecticide containing the 4A or 28 mode of action is recommended per crop, regardless of target pest. If two applications are made, they should be confined to the same five-week treatment window to avoid applying the same mode of action to successive generations of whiteflies.
Chlorantraniliprole = rynaxypyr (Coragen®) is a diamide insecticide used to manage caterpillar pests on tomato and other horticultural crops. Coragen is also used for leafminer control. Durivo® (soil application) and Voliam Flexi® (foliar) contain chlorantraniliprole and thiamethoxam, the same active ingredients as Coragen and Platinum respectively. With the registration of Verimark and Exirel, diamide insecticides are now available to target pests of tomato at each stage of development: nursery, at-plant, vegetative and fruiting. The risk is high that SWF and other pests of tomato will develop resistance to diamide insecticides if they are overused. Growers using Verimark for early season protection against SWF and TYLCV should not use Group 28 insecticides for management of leafminer and caterpillars in the same crop or at a minimum should avoid the use of this mode of action for at least five weeks after the application of Verimark. Other options with different modes of action are available for control of worm pests if needed.
Biotype Q of the SWF, also known as Bemisia tabaci MED, is prevalent in the Mediterranean region and has demonstrated the ability to develop even higher levels of resistance than the B biotype to many of the commonly used insecticides for managing whiteflies, including the pyrethroids, neonicotinoids, pymetrozine and insect growth regulators (Courier and Knack). Resistance in biotype Q is more stable than in biotype B, i.e. resistance appears not to diminish even in the absence of insecticide exposure. Biotype Q has been found in greenhouses and nurseries in many states including Florida, where since 2016 it has also been detected on landscaping plants in residential areas. It has not yet been detected in significant numbers in the field in Florida. Nevertheless, it represents a potential threat to vegetables and other crops in Florida and is another reason why resistance management guidelines should be strictly adhered to. These include rotation of insecticide modes of action as well as crop hygiene and cultural controls mentioned below.
Recommendations
Crop Hygiene
Field hygiene should be a high priority and should be an integral part of the overall strategy for managing whitefly populations, TYLCV incidence, and insecticide resistance. These practices will help reduce the onset of the initial infestation of whitefly, regardless of biotype, and lower the initial infestation level during the cropping period.
- Establish a minimum 2-month crop-free period during the summer, preferably from mid-June to mid-August in south and south central Florida.
- Disrupt the virus-whitefly cycle in winter by creating a break in time and/or space between fall and spring crops, especially tomato.
- Destroy crops quickly and thoroughly after harvest, killing whiteflies and preventing re-growth.
- Promptly and efficiently destroy all vegetable crops within 5 days of final harvest to decrease whitefly numbers and sources of plant viruses like TYLCV.
- Use a contact desiccant ("burn down") herbicide in conjunction with a heavy application of oil (not less than 3% emulsion) and a non-ionic adjuvant to destroy crop plants and to kill whiteflies quickly.
- Time burn down sprays to avoid crop destruction during windy periods, especially when prevailing winds are blowing whiteflies toward adjacent plantings.
Destroy crops block by block as harvest is completed rather than waiting and destroying the entire field at one time.
Pre-Plant Cultural Control Practices
Reduce overall whitefly populations and avoid introducing whiteflies and TYLCV into crops by strict adherence to good cultural practices.
- Plant whitefly and virus-free transplants.
- Use transplants grown in isolation from production fields.
- Inspect transplants for whiteflies and other pests and diseases
- Delay planting new fall crops as long as possible.
- Do not plant new crops near or adjacent to old, infested crops, especially of tomato but also of other whitefly sources such as cucurbits or possible sources of TYLCV like pepper or beans.
- Use determinant varieties of grape tomatoes to avoid extended cropping season.
- Use TYLCV-tolerant tomato cultivars where possible and appropriate, especially during historically critical periods of high virus pressure. Continue whitefly control even on TYLCV-tolerant cultivars which can still host the virus and are subject to tomato irregular ripening.
Use UV-reflective (metalized) mulch on plantings that are historically most commonly infested with whiteflies and infected with TYLCV.
Post-Planting Practices
- Scout for whitefly adults and apply a short reentry interval insecticide if necessary prior to cultural manipulations such as pruning, tying, etc.
- Rogue tomato plants with symptoms of TYLCV at least until second tie.
- Plants should be treated for whitefly adults prior to rogueing and, if nymphs are present, should be removed from the field, preferably in plastic bags, left in the sun and then disposed of as far from production fields as possible.
- Manage weeds within crops to minimize interference with spraying
- Dispose of cull tomatoes as far from production fields as possible.
- If deposited in pastures, fruit should be spread instead of dumped in a large pile to encourage consumption by cattle. The fields should then be monitored for germination of tomato seedlings, which should be controlled by mowing or with herbicides.
- Plants should be treated for whitefly adults prior to rogueing and, if nymphs are present, should be removed from the field, preferably in plastic bags, left in the sun and then disposed of as far from production fields as possible.
- Avoid u-pick or pin-hooking operations unless effective whitefly control measures are continued.
- Destroy old crops within 5 days after last harvest; destroy whitefly-infested abandoned crops, and control volunteer plants with a desiccant herbicide and oil.
- Plant non-host cover crops such as Sudex during summer fallows or rye grass during winter to discourage weeds and volunteer crop plants from growing and being infested by whiteflies.
Insecticidal Control Practices
- Delay resistance to neonicotinoid and other insecticides by using a proper whitefly insecticide program. Follow the label!
- Apply a neonicotinoid one time to transplants in the production facility, 7-10 days before shipping. Use products in other chemical classes, including Fulfill, soap, etc. before this time.
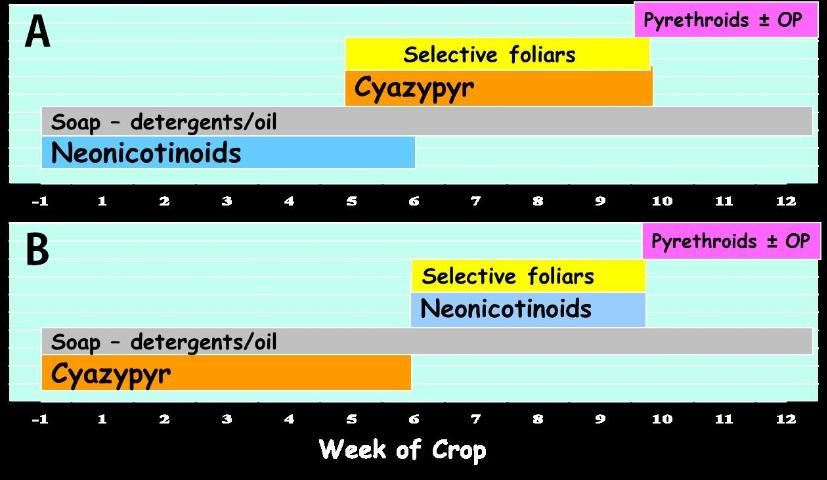
- Use a soil application containing a neonicotinoid (group 4A) or cyantraniliprole (group 28) no more than once each during a single crop (Figure 4).
- Do not repeat with a foliar application of either mode of action. If only foliar applications of these insecticides are to be made, then restrict each mode of action to a single 5-week period within any crop cycle.
- As control of whitefly nymphs diminishes following soil applications, use rotations of insecticides of other chemical classes as needed based on scouting recommendations. (Figure 4).
- Consult the Cooperative Extension Service for the latest recommendations.
- Use selective rather than broad-spectrum control products where possible to conserve natural enemies and enhance biological control.
- Do not apply insecticides on weeds on field perimeters.
These could kill whitefly natural enemies, and thus interfere with biological control, as well as select for biotype Q, if present, which is more resistant to many insecticides than biotype B.
Two possible programs for insecticidal whitefly control: A: neonicotinoid or butenolide (Sivanto Prime) drench just prior to and directly after planting followed later in the crop cycle by a soil application of cyantraniliprole (cyazypyr) or by foliar applications of selective products mid-season, finishing with one or more pyrethroid sprays with or without an organophosphate (malathion) at the end of the season if necessary to reduce whitefly migration to other crops. Products such as soaps, oils or biologicals could be used any time as needed. B. Reversing the order of cyazypyr and neonicotinoid/butenolide soil applications.
Efficacy indications generated by Florida researchers for insecticides labeled for use against whitefly on tomato in Florida and other relevant information are summarized and tabulated in Tables 1 and 2 by mode of action to facilitate planning a rotation program. IRAC (Insecticide Resistance Action Committee) provides additional information on general resistance management and on resistance classification of specific insecticides at its website: http://www.irac-online.org.
For Additional Information
Arnó, J., R. Gabarra, T-X Liu, A.M. Simmons, D. Gerling. 2010. Natural Enemies of Bemisia tabaci: Predators and Parasitoids. In PA Stansly and SE Naranjo [Eds.], Bemisia, Bionomics and Management of a Global Pest. Springer, Dordrecht. pp 385-421
Caballero, R. S. Cyman and D. J. Schuster. 2011. Monitoring Insecticide Resistance in Biotype B of Bemisia tabaci (Hemiptera: Aleyrodidae) in Florida. Florida Entomologist 96(4):1243-1256.
Castle S.J, J. C. Palumbo N. Prabhaker, A. R. Horowitz, I. Denholm. 2010. Ecological determinants of Bemisia tabaci resistance to insecticides. In P.A Stansly and S.E Naranjo [Eds.], Bemisia, Bionomics and Management of a Global Pest. Springer, Dordrecht pp 423–466
De Barro, P.J., S. S. Lui, S.S., L. M. Boykin, and A. B. Disndale. 2011. Bemisia tabaci: A Statement of Species Status. Annu. Rev. Entomol. 56: 1-19.
Horowitz, A. R., Y. Antignus, D. Gerling. Management of Bemisia tabaci Whiteflies. IN: The Whitefly, Bemisia tabaci (Homoptera: Aleyrodidae) Interaction with Geminivirus-Infected Host Plants, Thomson, WMD [Ed.]. pp 293-322.
McAuslane, H. J. 2009. Sweetpotato Whitefly B Biotype of Silverleaf Whitefly, Bemisia tabaci (Gennadius) or Bemisia argentifolii Bellows and Perring (Insecta: Hemiptera: Aleyrodidae) IPN: EENY129. Gainesville: Institute Food and Agricultural Sciences. from https://edis.ifas.ufl.edu/in286
McKenzie, C. L., G. Hodges, L. S. Osborne, F. J. Byrne, and R. G. Shatters. 2009. Distribution of Bemisia tabaci biotypes in Florida- Investigating the Q invasion. J. Econ. Entomol. 102(2): 670-676.
Pernezny, K., D. Schuster, P. Stansly, G. Simone, V. Waddill, J. Funderburk, F. Johnson, R. Lentini, and J. Castner. 1996. Florida tomato scouting guide with insect and disease identification keys. Univ. of Florida, IFAS, Florida Coop. Ext. Serv., SP-22. 45 pp. https://erec.ifas.ufl.edu/featured-3-menus/extension/florida-tomato-scouting-guide/
Polston, J. E., L. Cohen, T. A. Sherwood, R. Ben-Joseph, and M. Lapidot. 2006. Capsicum species: symptomless hosts and reservoirs of Tomato yellow leaf curl. Phytopathology 96: 447-452.
Polston, J. E., D.J. Schuster, and J.E. Taylor. 2009. Identification of weed reservoirs of Tomato yellow leaf curl virus in Florida. pp. 32-33. In E. Simone, C. Snodgrass and M.Ozores-Hampton [eds.] Florida Tomato Institute Proceedings. Naples, FL, USA. University of Florida Institute of Food and Agricultural Sciences, Gainesville, FL.
Schuster, D. J., R. Mann and P. R. Gilreath. 2006. Whitefly resistance update and proposed mandated burn down rule, pp. 24-28. In P. Gilreath and K. Cushman [eds.], 2006 Fla. Tomato Institute Proc., Univ. Fla., PRO 523.
Schuster, D. P. Stansly, J. Polston and P. Gilreath. Management of Whiteflies, TYLCV and Insecticide Resistance. http://ipm.ifas.ufl.edu/resources/success_stories/T&PGuide/pdfs/Chapter4/Whitefly_Mgmt.pdf
Smith, H. A and C. A. Nagle. 2014. Susceptibility of Bemisia tabaci to group 4 insecticides. Pp. 27-28. In M. Ozores-Hampton and C. Snodgrass [eds.], Fla. Tomato Institute Proc., University of Florida, IFAS Extension. http://swfrec.ifas.ufl.edu/docs/pdf/veg-hort/tomato-institute/proceedings/ti14_proceedings.pdf.
Stansly, P. A., and E. T. Natwick. 2010. Integrated systems for managing Bemisia tabaci in protected and open field agriculture, pp. 467-497. In PA Stansly and SE Naranjo [Eds.], Bemisia, Bionomics and Management of a Global Pest. Springer, Dordrecht.
Stansly, PA, Ozores-Hampton M, Kostyk B. 2011. Insecticides and Resistant Varieties for Management of Whiteflies and TYLCV. In M. Ozores-Hampton and C. Snodgrass [eds.], Proceedings: Florida Tomato Institute Vegetable Crops Special Series. IFAS, Gainesville, FL. pp 10-15. http://swfrec.ifas.ufl.edu/docs/pdf/veg-hort/tomato-institute/proceedings/ti11_proceedings.pdf
Webb, S.E., D.J. Schuster, P.A. Stansly, J.E. Polston, S. Adkins, C. Baker, P. D. Roberts, O. Liburd, T. Nyoike, E. McAvoy, and A. Whidden. 2011. Recommendations for Management of Whiteflies, Whitefly-transmitted viruses, and Insecticide Resistance for Production of Cucurbit Crops in Florida. https://edis.ifas.ufl.edu/in871
Mode of action (MOA), active ingredient, trade name, minimum reentry interval (REI), days to harvest (PHI) and other pests controlled by insecticides labeled for soil application to tomato for control of sweetpotato whitefly in Florida.