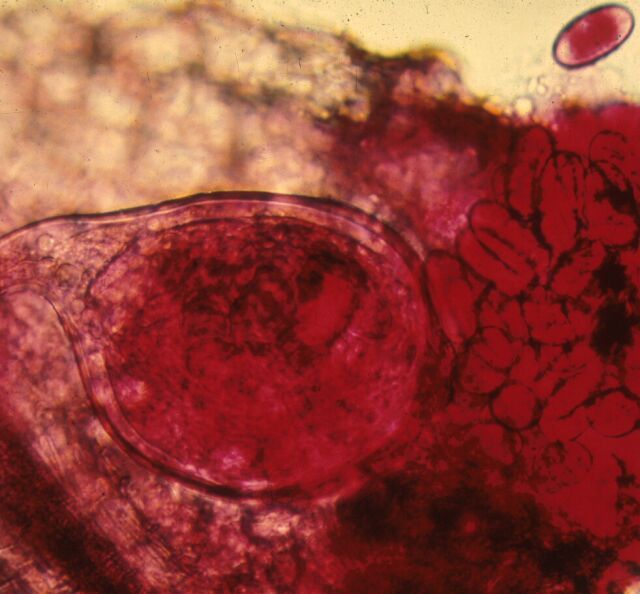
Plant parasitic nematodes are small microscopic roundworms that live in the soil and attack the roots of plants. Crop production problems induced by nematodes, therefore, generally occur as a result of root dysfunction, reducing rooting volume and foraging and utilization efficiency of water and nutrients. Many different genera and species of nematodes can be important to crop production in Florida. In many cases a mixed community of plant parasitic nematodes is present in a field, rather than having a single species occurring alone. In general, the most widespread and economically important nematode species include the root-knot nematode, Meloidogyne spp., and sting nematode, Belonolaimus longicaudatus. The host range of these nematodes, as with others, includes many different weeds and most if not all of the commercially grown vegetables within the state. Yield reductions can be extensive but vary significantly between plant and nematode species. In addition to the direct crop damage caused by nematodes, many of these species have also been shown to predispose plants to infection by fungal (Figure 1) or bacterial pathogens or to transmit virus diseases, which contribute to additional yield reductions.

Biology and Life History
Most species of plant parasitic nematodes have a relatively simple life cycle consisting of the egg, four larval stages, and the adult male and female. Development of the first stage larvae occurs within the egg where the first molt occurs. Second stage larvae hatch from eggs to find and infect plant roots or in some cases foliar tissues. Host finding or movement in soil occurs within surface films of water surrounding soil particles and root surfaces. Depending on species, feeding will occur along the root surface or in other species like root-knot, young larval stages will invade root tissue, establishing permanent feeding sites within the root. Second stage larvae will then molt 3 times to become adult male or female. For most species of nematodes, as many as 50-100 eggs are produced per female, while in others such as root-knot, upwards of 2000 may be produced (Figure 2). Under suitable environmental conditions, the eggs hatch and new larvae emerge to complete the life cycle within 4 to 8 weeks depending on temperature. Nematode development is generally most rapid within an optimal soil temperature range of 70 to 80°F.
Symptoms
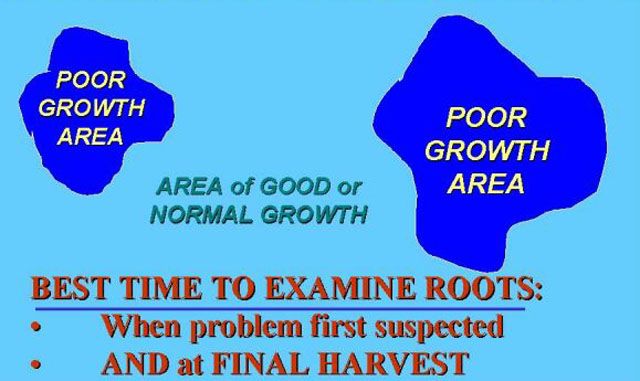
Typical symptoms of nematode injury can involve both aboveground and below ground plant parts. Foliar symptoms of nematode infestation of roots generally involve stunting and general unthriftiness, premature wilting (Figure 3) and slow recovery to improved soil moisture conditions, leaf chlorosis (yellowing) (Figure 4), and other symptoms characteristic of nutrient deficiency. An increased rate of ethylene production, thought to be largely responsible for symptom expression in tomato, has been shown to be closely associated with root-knot nematode root infection and gall formation. Plants exhibiting stunted or decline symptoms usually occur in patches of nonuniform growth rather than as an overall decline of plants within an entire field (Figure 5).



The time in which symptoms of plant injury occur is related to nematode population density, crop susceptibility, and prevailing environmental conditions. For example, under heavy nematode infestation, crop seedlings or transplants may fail to develop, maintaining a stunted condition, or die, causing poor or patchy stand development (Figure 6). Under less severe infestation levels, symptom expression may be delayed until later in the crop season after a number of nematode reproductive cycles have been completed on the crop. In this case aboveground symptoms will not always be readily apparent early within crop development, but with time and reduction in root system size and function, symptoms become more pronounced and diagnostic.

Root symptoms induced by sting or root-knot nematodes can oftentimes be as specific as aboveground symptoms. Sting nematode can be very injurious, causing infected plants to form a tight mat of short roots, oftentimes assuming a swollen appearance. New root initials generally are killed by heavy infestations of the sting nematode, a symptom reminiscent of fertilizer salt burn. Root symptoms induced by root-knot cause swollen areas (galls) on the roots of infected plants (Figure 7). Gall size may range from a few spherical swellings to extensive areas of elongated, convoluted, tumorous swellings which result from exposure to multiple and repeated infections. Symptoms of root galling can in most cases provide positive diagnostic confirmation of nematode presence, infection severity, and potential for crop damage.

Damage
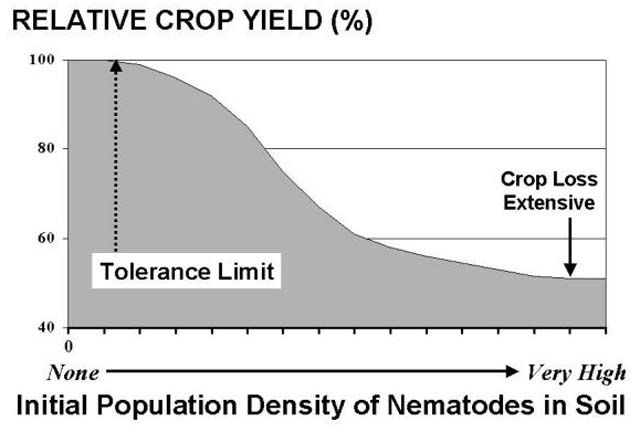
For most crop and nematode combinations the damage caused by nematodes has not been accurately determined. Most vegetable crops produced in Florida are susceptible to nematode injury, particular by root-knot and sting nematodes. Plant symptoms and yield reductions are often directly related to preplant infestation levels in soil and to other environmental stresses imposed upon the plant during crop growth (Figure 8). As infestation levels increase so then do the amount of damage and yield loss. In general, the mere presence of root-knot or sting nematodes suggests a potentially serious problem, particularly on sandy ground during the fall when soil temperatures favor high levels of nematode activity and reproduction. At very high levels, typical of those that might occur under doubling cropping, plants may be killed. Older transplants, unlike direct seed, may tolerate higher initial population levels without incurring as significant a yield loss.
Field Diagnosis and Sampling
Because of their microscopic size and irregular field distribution, soil and root tissue samples are usually required to determine whether nematodes are causing poor crop growth or to determine the need for nematode management. For nematodes, sampling and management are a preplant or postharvest consideration because if a problem develops in a newly planted crop, there are currently no postplant corrective measures available to rectify the problem completely once the nematode becomes established. Nematode density and distribution within a field must therefore be accurately determined before planting, guaranteeing that a representative sample is collected from the field. Nematode species identification is currently only of practical value when rotation schemes or resistant varieties are available for nematode management. This information must then be coupled with some estimate of the expected damage to formulate an appropriate nematode control strategy.
Advisory or Predictive Sample (Prior to Planting)
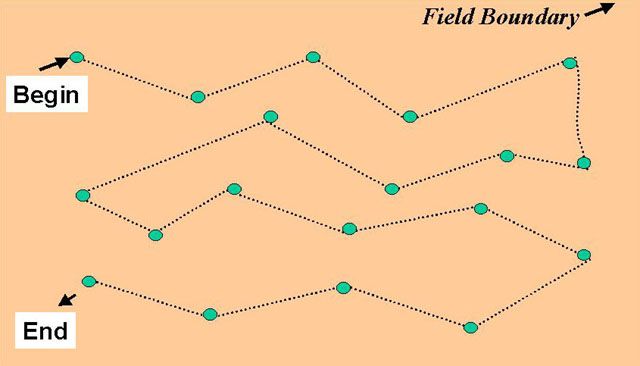
Samples taken to predict the risk of nematode injury to a newly planted crop must be taken well in advance of planting to allow for sample analysis and treatment periods if so required. For best results, sample for nematodes at the end of the growing season, before crop destruction, when nematodes are most numerous and easiest to detect. Collect soil and root samples from 10 to 20 field locations using a cylindrical sampling tube, or, if unavailable, a trowel or shovel (Figure 9). Since most species of nematodes are concentrated in the crop rooting zone, samples should be collected to a soil depth of at least 6 to 10 inches. Plant-pathogenic nematodes can be deeply distributed throughout the soil profile well below the typical sampling depth and zones of root growth, and have the capability to move upward to infest plant roots. In this scenario, samples procured from surface soil horizons may not adequately describe nematode populations and potential threats to crop growth and yield. As a practical matter, however, sample in a regular pattern over the area, emphasizing removal of samples across rows rather than along rows (Figure 10). One sample should represent no more than 10 acres for relatively low-value crops and no more than 5 acres for high-value crops. Fields that have different crops (or varieties) during the past season or that have obvious differences either in soil type or previous history of cropping problems should be sampled separately. Sample only when soil moisture is appropriate for working the field, avoiding extremely dry or wet soil conditions.

Diagnostics on Established Plants
Roots and soil cores should be removed to a depth of 6 to 10 inches from 10 to 20 suspect plants. Avoid dead or dying plants, since dead or decomposing roots will often harbor few nematodes. For seedlings or young transplants, excavation of individual plants may be required to insure sufficient quantities of infested roots and soil. Submission of additional samples from adjacent areas of good growth should also be considered for comparative purposes (Figure 11).
For either type of sample, once all soil cores or samples are collected, the entire sample should then be mixed thoroughly but carefully, and a 1 to 2 pint subsample removed to an appropriately labeled plastic bag. Remember to include sufficient feeder roots. The plastic bag will prevent drying of the sample and guarantee an intact sample upon arrival at the laboratory. Never subject the sample(s) to overheating, freezing, drying, or to prolonged periods of direct sunlight. Samples should always be submitted immediately to a commercial laboratory or to the University of Florida Nematode Assay Laboratory for analysis. If sample submission is delayed, then temporary refrigerated storage at temperatures of 40 to 60°F is recommended.
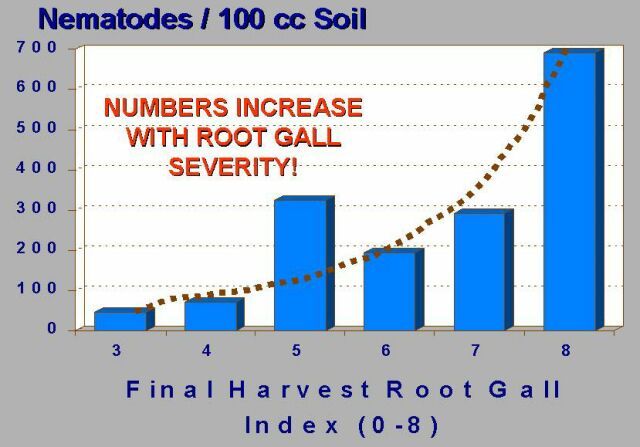
Recognizing that the root-knot nematode causes the formation of large swollen areas or galls on the root systems of susceptible crops, relative population levels and field distribution of this nematode can be largely determined by simple examination of the crop root system for root gall severity (Figure 12). Root gall severity is a simple measure of the proportion of the root system that is galled. Immediately after final harvest, a sufficient number of plants should be carefully removed from soil and examined to characterize the nature and extent of the problem within the field. In general, soil population levels increase with root gall severity (Figure 13). This form of sampling can in many cases provide immediate confirmation of a nematode problem and allows mapping of current field infestation. As inferred previously, the detection of any level of root galling usually suggests a nematode problem for subsequent planting of a susceptible crop, particularly within the immediate areas from which the galled plant(s) were recovered.

General Management Considerations
Currently nematode management considerations include crop rotation of less susceptible crops or resistant varieties, cultural and tillage practices, use of transplants, and preplant nematicide treatments. Where practical, these practices are generally integrated into the summer or winter 'off-season' cropping sequence. It should be recognized that not all land management and cultural control practices are equally effective in controlling plant parasitic nematodes and varying degrees of nematode control should be expected. These methods, unlike other chemical methods, tend to reduce nematode populations gradually through time. Farm specific conditions, such as soil type, temperature, and moisture, can be very important in determining whether different cultural practices can be effectively utilized for nematode management.
Cultural Practices
Crop Rotation
For crop rotation to be effective, crops unsuitable for nematode infection, growth, or reproduction must be introduced into the rotation sequence. In most of Florida it is not uncommon to observe a multispecies community of nematodes all occurring within the same field. Under these circumstances it may not be possible to find a rotation or cover crop that will effectively reduce populations of all nematode pests, particularly if root-knot and sting nematodes occur in combination. In this case, crop rotations detrimental to root-knot, which is generally the most difficult to control, should be selected. In some cases, resistant crop varieties are available that can be used within the rotation sequence to minimize problems to some species of root-knot but not sting nematodes.
Use of poor or nonhost cover crops within the rotation sequence, may in some cases offer an effective approach to nematode control. Four leguminous cover crops adaptable for managing soil populations of sting or root-knot nematode include Iron Clay Cowpea (Vigna unguiculata . cv. 'Iron Clay'), sunn hemp (Crotalaria juncea), hairy indigo (Indigofena hirsuta) and American jointvetch (Aeschynomene americana). Sorghum is also a popular cover crop restoring large amounts of soil organic matter. It is a good host for sting nematode but not root-knot. Most of the small grains commonly used as winter cover crops in central and north Florida, such as rye, barley, wheat, or oats, can support limited reproduction of root-knot nematode. To avoid an increase in root-knot populations, these crops should only be planted when soil temperatures are below 65°F, a threshold temperature for nematode activity. Cover crop rotations with some pastureland grasses (particularly pangola digitgrass, and to some extent bahiagrass, and Bermuda grass) have significantly reduced, but not eliminated, root-knot nematodes. In north Florida, long term (6- to 9-year) pastureland rotations have allowed economic melon production within root-knot infested fields. It should be recognized that as the crop rotation period is shortened or eliminated, nematode problems will intensify accordingly. Other perennial legumes currently under evaluation may play an important role in future nematode management programs.
For cover crops to be most effective stands must be established quickly and undesirable weeds that can serve as alternative hosts must be controlled. Given that many different weeds serve as alternative plant hosts to nematodes (i.e., nutsedges), it may not be possible to manage root-knot nematode with crop rotation unless an integrated program to manage weeds is also considered and implemented within the field. With many cover crops, rapid stand establishment has been a significant problem. Similarly, economic crop rotation sequences are often further complicated by lack of crop management skills, specialized equipment to grow and harvest the crop, or by the lack of closely located processing facilities or markets. In some cases other measures should be considered such as fallowing, which is usually as efficient as crop rotation for reducing field infestations of nematodes.
Fallowing
Clean fallow during the off-season is probably the single most important and effective cultural control measure available for nematodes. When food sources are no longer readily available, soil population densities of nematodes gradually decline with death occurring as a result of starvation. Due to the wide host range of many nematode species, weeds and crop volunteers must be controlled during the fallow period to prevent nematode reproduction and further population increase. At least two discing operations are generally required to maintain clean fallow soil conditions during the interim period between crops (Figure 14). Fallowing by use of herbicides to deplete nematode populations is a much slower process because the soil is not disturbed, thereby subjecting nematodes from deeper soil layers to the drying action of sun and wind. The unfavorable effects of fallowing on soil organic matter and soil structure is usually more than compensated for by the level of nematode control achieved and the resulting increase in crop productivity. When soil erosion is a potentially serious problem other measures should be considered.

Biological Control
At present there are no effective, commercially available, biological control agents that can be successfully used to control nematodes.
Plant Resistance
Use of nematode-resistant crop varieties has not been extensively evaluated in Florida, but is often viewed as the foundation of a successful integrated nematode management program on all high value crops in which soil fumigants are currently used. Commercially available nematode-resistant varieties are currently available only for tomato, pepper, southernpea, and sweet potato. In a resistant variety, nematodes fail to develop and reproduce normally within root tissues, allowing plants to grow and produce fruit even though nematode infection of roots occurs. Some crop yield loss can still occur, however, even though the plants are damaged less and are significantly more tolerant of root-knot infection than that of a susceptible variety. Any crop varieties with the code VFN (Verticillium, Fusarium, Nematodes) on the seed packet or label are resistant to common root-knot nematode species. Although even resistant tomato varieties can still exhibit some root galling under high nematode levels, they usually maintain their yield.
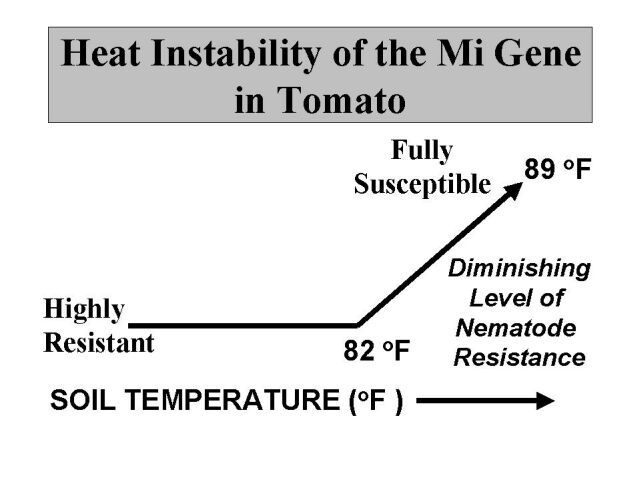
In tomato, a single dominant gene (subsequently referred to as the Mi gene) has been widely used in plant breeding efforts and varietal development, which confers resistance to all of the economically importance species of root-knot nematode found in Florida, including Meloidogyne incognita, arenaria, and javanica. Commercially resistant fresh market varieties, climatically and horticulturally adapted for Florida, are available in a number of tomato varieties (e.g, 'Sanibel'). Unfortunately, in previous research with resistance tomato varieties, the resistance has often failed as a result of the heat instability or apparent temperature sensitivity of the resistant Mi gene (Figure 15). For example, previous research has demonstrated threshold soil temperatures and incremental reductions in nematode resistance with each degree above 78°F, such that at 91°F tomato plants are fully susceptible. This would suggest that, in Florida, use of these varieties may have to be restricted to spring plantings when cooler soil temperatures prevail. In addition to the failure of the Mi gene to confer resistance at high soil temperature, Mi-virulent nematode isolates capable of reproduction and causing plant damage have been identified in many areas of the world. All too frequently, the discovery is made that field populations of many different species of root-knot nematode are present, and those species outside of the resistance proliferate and cause damage.
In pepper, two newly developed root-knot nematode resistant varieties (Carolina Belle and Carolina Wonder) were released from the USDA Vegetable Research Laboratory for commercial seed increase in April 1997. Both varieties are open pollinated and homozygous for the N root-knot nematode resistant gene. Preliminary research has demonstrated that these varieties confer a high degree of resistance to the root-knot nematode; however, expression of resistance is heat sensitive. Further research is necessary to characterize the usefulness of these varieties under the high soil temperature conditions of Florida. Like tomato, use of these varieties may have to be restricted to spring plantings when cooler soil temperatures prevail.
In addition to problems of heat instability, the continuous or repeated planting of resistant plant varieties will almost certainly select for virulent races of Meloidogyne capable of overcoming the resistance. Therefore the duration and/or utility of the resistance may be time-limited. In previous studies with resistant tomatoes, resistance breaking nematode races have been shown to develop within 1 to 3 years. Since new races of the nematode can develop so rapidly, a system of integrated control usually mandates the rotation of resistant and non-resistant varieties to slow the selection process for new virulent races. Previous trials in Florida have demonstrated the capacity of some species or races of root-knot to reproduce and inflict damage upon a resistant tomato variety. The results of these experiments have demonstrated that even with a resistant variety, which was damaged less than that of a susceptible variety, some consideration of initial soil population levels of the root-knot nematode must be observed to minimize tomato yield losses. Given that significant yield losses can still occur, combined efforts to manage soil populations to low levels prior to planting must still be considered, particularly if tomatoes are planted as a fall crop. If this situation develops, the combination of a nematicide and resistant variety may also be an option to reduce nematode populations to nondamaging levels.
With the use of appropriate rootstocks, grafting that uses tomato hybrids (Solanum lycopersicum L.) and interspecific tomato hybrids (S. lycopersicum· x S. habrochaites) have been used worldwide as disease-resistant rootstocks in grafted tomato production. When resistant tomato cultivars with market-required characteristics (e.g. fruit type, size, flavor or yield) are not available, market-acceptible but nematode-susceptible cultivars can be grafted onto nematode-resistant rootstocks. In this scenario, grafting can be a useful technique for Florida vegetable growers to overcome soilborne pathogens and for improving nematode control in the absence of soil fumigants. Root-knot nematode resistance has not yet been identified in some crops, but hybrids have been proven to be good candidates for root-knot nematode-resistant rootstocks suitable for grafting onto commercial cultivars. Most of the current grafting research examines the use of the Mi resistance gene incorporated into these rootstocks as a tool to manage root-knot nematode. For organic production, these hybrid rootstocks have all performed similarly and have significantly reduced root galling of tomato compared with the non-grafted plants. More studies are warranted to examine the influence of these interspecific hybrid rootstocks on the yield of both determinant and indeterminate cultivars grown in field production systems. When assessing whether to use grafted tomato plants for root-knot nematode management, growers need to consider the severity of the nematode infestation, the growing system, and the scion and rootstock cultivars to be used. Grafting vegetable cultivars onto resistant rootstocks appears to have potential as a practical component of a systems approach for root-knot nematode control under Florida field conditions.
Soil Amendments
Many different types of amendments and composted materials have been applied to soil to suppress populations of plant parasitic nematode and improve crop yield and plant health. Animal manures, poultry litter, and disk-incorporated cover crop residues are typical examples of soil amendments used in agriculture to improve soil quality and as a means for enhancing biocontrol potential of soil. Some amendments that contain chitin and inorganic fertilizers that release ammoniacal nitrogen into soil suppress nematode populations directly and enhance the selective growth of microbial antagonists of nematodes. More recently, composted municipal wastes and sludges have been used to amend soil to improve soil fertility, organic matter content, water-holding capacity, nutrient retention, and cation exchange capacity.
Suppression of soilborne pathogens via the incorporation or simple mulching of composted amendments is reputedly based on enhanced microbial activity and increased numbers of antagonists generated by decomposition of the amendment in soil. Soils with a diversity of beneficial microorganisms are more suppressive to pathogens than soils with little or no biological diversity. Other possible mechanisms for pathogen suppression by composts include direct inhibition of the pathogen or reduced infectivity of the organisms into the plant host. Population increases of beneficial organisms in soil appear to be the direct result of environmental changes brought about by the amendments after addition to soil. This suggests that to sustain soil suppressiveness, amendments must be periodically reapplied to maintain the soil environment conducive to antagonists.
The level to which soilborne pest and disease control can be achieved is not only related to the type of material but to the age of the compost. Nematode and disease suppression has been repeatedly demonstrated with composted municipal yard wastes containing significant quantities of tree bark. If the compost is immature, the product may not only be difficult to handle and have an offensive odor, but may contain salts and metabolites toxic to plants. For example, weed suppression has been demonstrated with some types of immature composted materials that contain and or produce organic acids with phytotoxic properties. Other studies have shown that soils amended with different sources of composted municipal wastes were disease suppressive as the long as they were relatively fresh (< 6 months), but as the composted municipal waste was aged, disease suppressiveness was lost. In other Florida studies, application of composted municipal wastes at rates up to 120 tons per acre have not been shown to be pesticidal in activity, but actually dramatically increased populations of nematodes and other disease organisms such as Fusarium and Phytopthora spp. Nematode population increases were directly related to increases in plant growth and root system size with amendment application rate.
Previous studies in Florida were conducted to determine the extent to which increasing application rates of a municipal solid composted waste affect the ability of tomato plants to tolerate root infection by species of root-knot nematode (Meloidogyne spp.) These studies showed that in a sandy soil poor in organic matter content (less than 2%), tomato yields could be increased significantly with soil amendments in both nematode free or nematode infested soil. The impact of the root-knot nematode on tomato yield was effectively constant, however, suggesting that application of the soil amendment did not enhance the ability of tomato plants to tolerate infection by the root-knot nematode. Much of the previous and ongoing research in Florida also seems to indicate that the major effects of soil amendments to crop yields appear to be less related to nematode or soil pathogen control than to enhanced plant nutrition and nutrient and water availability.
It is not clear at this time and preliminary stage of UF/IFAS field research whether benefits to crop growth after the initial crop following soil amendment application can be expected. Previous studies showed no response in second-crop tomato yields (double crop) following amendment application rates from 15 to 120 tons per acre. Disappearance of nutrients and soil organic matter content appears to be very rapid in the hot, moist soils of Florida. Preliminary research suggests that reapplication of the amendments may have to be made on 1-2 year basis to sustain crop growth and yield benefits. In summary, the high rates of application (tons/acre) and attendant costs required for crop response and nematode control for many different types of organic amendments, and the apparently rapid losses of the materials in soil appear to preclude use of these materials primarily to homeowner or small farm operations at this time. However, with additional research and advances in application technology and use efficiency, use of soil amendments may become an integral component of Florida crop production systems.
Flooding
Flooding has been shown to suppress nematode populations (Figure 16). Alternating 2 to 3 week cycles of flooding and drying have proven to be more effective than long, continuous flooding cycles. At present, only limited areas within the state are situated to take advantage of flooding as a viable means of nematode control. Given the growing concern about aquifer depletion, salt water intrusion, and water use inefficiencies, it seems unlikely that Florida water management officials will continue to permit flooding (other than natural flooding) within these areas in the future.

Soil Solarization
Soil solarization is a nonchemical technique in which transparent polyethylene tarps are laid over moist soil for a 6 to 12 week period to heat noncropped soils to temperatures lethal to nematodes and other soil-borne pathogens (Figure 17). Soil temperatures are magnified due to the trapping of incoming solar radiation under the clear, polyethylene panels. To be effective, soils must be wetted and maintained at high soil moisture content to increase the susceptibility (thermal sensitivity) of soil borne pests and thermal conductivity of soil. Wet mulched soils increase soil temperatures due primarily to the elimination of heat loss by evaporation and upward heat convection, in addition to a greenhouse effect by prohibiting dissipation of radiation from the soil. At the end of the solarization period the clear plastic is painted with a white latex paint to allow continued use of the plastic as a mulch cover for the production of vegetables on raised beds.

The most successful use of soil solarization appears to occur in heavier (loamy to clay soils) rather than sandy soils. Soils with poor water holding capacity and rapid drainage can significantly inhibit heat transfer to deeper soil horizons. Loss of pest control is directly correlated with soil depth. The depth to which lethal temperature can be achieved (6 to 8 inches) is also dependent on the intensity and duration of sunlight and ambient temperature. At present, the only time to consider soil solarization for pest control is during our hot, summer and early fall months, which fortunately are "off-season" in most peninsular Florida vegetable row crops. Unfortunately our summers are also our wettest period of the year with frequent afternoon rain showers that have a cooling effect on the soil.
Many different pests have been suppressed and or controlled by soil solarization, particularly within arid environments with intense sunshine and limited cloud cover and rainfall. Previous studies in Florida have demonstrated that soil solarization can also be effective in a subtropical environment. Plant parasitic nematodes have generally proved to be more difficult to control with soil solarization, as have some weed pests such as crabgrass in a central Florida study. The results of preliminary experiments are also suggesting the potential for selection pressures towards a buildup of heat tolerant individuals that may serve to reduce soil solarization efficacy after repeated use as a nematode control tactic. In some studies, effective use of solarization for nematode control has required an integrated systems approach, coupling solarization with other chemical or nonchemical approaches. For example, the combined use of soil solarization with a nematicide has improved nematode control and crop yield. In addition, use of virtually impermeable, photo-selective plastic mulches may also complement low dose fumigant treatments to reduce weed germination and growth in the event of extended periods of cloud cover occurring during the solarization regime. At this time, further research is needed demonstrating soil solarization pest control activity and consistency in the various geographical regions of Florida where vegetable crops are grown.
Other Cultural Practices
Other cultural measures that reduce nematode problems include rapid destruction of the infested crop root system following harvest. Fields that are disked as soon as possible after the crop is harvested will not only prevent further nematode population growth but subject existing populations to dissipation by sun and wind. Use of nematode free tomato transplants is also recommended since direct seeded plants are particularly susceptible since they are vulnerable to injury for a longer duration, during an early, but critical period of crop development. Since nematodes can be carried in irrigation water that has drained from an infested field, growers should avoid use of ditch or pond waters for irrigation or spray mixtures. In most cases, a combination of these management practices will substantially reduce nematode population levels but will rarely bring them below economically damaging levels. This is especially true of lands that are continuously planted to susceptible crop varieties. In these cases some form of pesticide assistance will still usually be necessary to improve crop production.
Chemical Control
Nonfumigant Nematicides
All of the nonfumigant nematicides (Table 1) currently registered for use in tomatoes, peppers and eggplant are soil applied, with the exception of Vydate, which can also be applied foliarly. Nimitz, a new nonfumigant nematicide that became commercially available in 2015, is still actively under assessment in field trial evaluations. All of the nonfumigant nematicides must be incorporated with soil or carried by water into soil to be effective. These compounds must be uniformly applied to soil, targeting the application toward the future rooting zone of the plant, where they will contact nematodes or, in the case of systemics, in areas where they can be readily absorbed and taken up into the plant. Placement and incorporation within the top 3 to 6 inches of soil should provide a zone of protection for seed germination and transplant establishment, and protect initial growth of plant roots from seeds or transplants. Most studies that have been performed in Florida and elsewhere to evaluate nonfumigant nematicides have not always been consistent, either for controlling intended pests or for obtaining consistent economic returns to the grower, particularly when compared with conventional preplant mulched fumigation with a broadspectrum fumigant nematicide (Figure 18). As the name implies, they are specific to nematodes, have little residual activity, and require integrated use of other cultural or chemical pest control measures to manage other weed and disease pests. Many are reasonably mobile and are readily leached in our sandy, low organic soils, thus requiring special consideration to irrigation practices and management.

Nematode management must be viewed as a preplant consideration because once root infection occurs and plant damage becomes visible it is generally not possible to resolve the problem completely so as to avoid potentially significant tomato yield losses. Previous experiments have been conducted to evaluate the extent to which tomato plant growth and yield could be 'rescued' from root-knot nematode via early detection and treatment by post plant applications of the nonfumigant nematicide, Vydate (Oxamyl). The results of these experiments clearly showed that it was not possible to completely resolve the problem and avoid tomato yield losses with post plant applications of Vydate. This was particularly obvious in tomato yield responses with foliar applications of Vydate attempting to resolve a soilborne problem. If an attempt is going to be made to rescue the crop, the sooner the nematode problem is recognized and soil applications of Vydate started, the greater the improvement in tomato yields relative to plants maintained nematode free.
Fumigant Nematicides
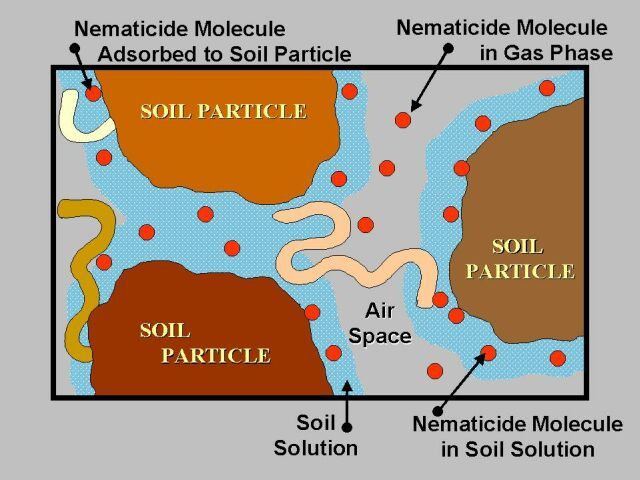
In Florida, use of broadspectrum fumigants (Table 2) effectively reduces nematode populations and increases vegetable crop yields, particularly when compared with nonfumigant nematicides. Since these products must diffuse through soil as gases to be effective (Figure 19), the most effective fumigations occur when the soil is well drained, in seedbed condition, and at temperatures above 60°F. Fumigant treatments are most effective in controlling root-knot nematode when residues of the previous crop are either removed or allowed to decay. When plant materials have not been allowed to decay, fumigation treatments may decrease but not eliminate populations of root-knot nematodes in soil, particularly nematodes within the egg stage. Crop residues infested with root-knot nematode may also shelter soil populations to the extent that significantly higher rates of application may be required to achieve nematode control. To avoid these problems, growers are advised to plan crop destruction and soil cultivation practices well in advance of fumigation to insure decomposition of plant materials before attempting to fumigate.
In general, the use of soil fumigants has been more consistently effective than non-fumigants for control of root-knot and sting nematodes in Florida. In the fine sands of Florida, dry soils (no more than or less than 12 to 15% available soil moisture content) are considered favorable for soil fumigation. In addition to buffer zones, the new fumigant labels also outline a new series of mandatory Good Agricultural Practices (GAP's) to reduce emissions and bystander exposures. These have become new mandatory label requirements effective January 1, 2011. The new GAPs that must be followed during all fumigant applications and that are posing Florida growers significant difficulties include changes in: 1) Soil Preparation: Soil shall be properly prepared and field trash must be properly managed. Residue from a previous crop must be worked into the soil to allow for decomposition prior to fumigation; 2) Soil Moisture: In general, the new soil fumigant labels will indicate that soil moisture in the top nine inches of soil must be between 50% to 80% of soil capacity (field capacity) immediately prior to fumigant application, subject to the exception where soil moisture must exceed field capacity to form a bed (e.g., certain regions in Florida where soil capacity may exceed the 80% allocated above). Following EPA reregistration, the new fumigant labels that went into effect December 1, 2012, detail additional use restrictions based on soil characteristics, buffer zones, requirements for Personal Protective Equipment (PPE), mandatory good agricultural practices (GAPs), applicator training certification, and other new rate modifying recommendations.
Since the phase out of methyl bromide began in 2005, many different alternative soil fumigants have been evaluated in field trials to characterize pest control efficacy and crop yield response (Table 3). The results of these research trials have provided basis for overall generalization of pesticidal activity for each of the alternative fumigant chemicals. As a standard for comparison, this research has repeatedly demonstrated methyl bromide to be very effective against a wide range of soilborne pests including nematodes, diseases, and weeds. Chloropicrin has proved very effective against diseases but seldom nematodes or weeds. Telone (1, 3-dichloropropene) is an excellent nematicide but generally performs poorly against weeds and diseases. Bacterial pathogens have not been satisfactorily controlled by any of the fumigants. Metam sodium and metam potassium can provide good control of weeds when placed properly in the bed; however, research to evaluate modification of rate, placement, and improved application technology have not resolved all problems of inconsistent pest control. Dimethyl disulfide (DMDS) has demonstrated good to excellent control of nematodes, disease, and weeds when coapplied with chloropicrin. Allyl isothiocyanate (AITC) (Dominus), the newest fumigant entry to be registered in Florida, has shown some promise for broadspectrum control of nematode, disease, and weeds but is still in comprehensive assessment for its relative pesticidal activity.
Generalized summary of maximum use rate and relative effectiveness of various soil fumigant alternatives to methyl bromide for nematode, soilborne disease, and weed control in Florida.
The phaseout of methyl bromide in the United States on January 1, 2015, has created a significant void for us in the Florida chemical arsenal used for soilborne pest and disease control. This fact is still made quite clear from a review of recent field research trials conducted in Florida that shows that no single, equivalent replacement (chemical or nonchemical) currently exists that exactly matches the broadspectrum efficacy of methyl bromide. In preparation for the complete phase-out and loss of methyl bromide in 2015, university research programs within Florida have continued to identify and evaluate more robust strategies that minimize cropping system impacts, accounting for a diverse range of pest pressures and environmental conditions. Based on summary and comparison of methyl bromide alternative chemical trial results in Florida, much of the current field research continues to focus on evaluations of chloropicrin co-applied with additional fumigants. In this co-application approach, chloropicrin has clearly been shown to be an integral, foundation component of any alternative chemical approach to replace methyl bromide. Of the chloropicrin combinations, Pic-Clor 60 and Telone C-35, a combination of 1,3-dichloropropene with either 60% or 35% chloropicrin, have been the most extensively evaluated in Florida field trials since 1994. Dimethyl disulfide (DMDS) in combination with chloropicrin (21%) has proven effective for soilborne pest and disease control in most Florida field trials. Coupling use of DMDS with totally impermeable mulch films (TIF), a new mandatory requirement for its use in Florida, has helped to resolve odor issues associated with its use, but has increased production costs accordingly. Allyl isothiocyanate, the newest registered fumigant in Florida, has looked promising in preliminary trials but it has not been extensively studied and as such should be considered "still in assessment." In general, this research has demonstrated that Pic Clor 60 or Telone C35, applied in combination with a separately applied herbicide or fumigant product for weed control, has been identified as the best chemical alternative replacement for methyl bromide for some vegetable row crops such as tomato and pepper. Recent research on soil application technologies in Florida and Georgia have demonstrated improved weed control with metam sodium or potassium applied through a series of minicoulters to the established plant bed just before installation of the plastic mulch. This has also been demonstrated in large scale, commercial field trials around the state. In many of these studies, use of any of the other alternative fumigants, except Pic Clor 60 or Telone C-35 in combination with a herbicide and or additional fumigant treatment, has not consistently produced the same near equivalent yields to those of methyl bromide with increasing pest pressure. Under conditions of high pest pressures (nematodes, disease), other chemicals (e.g., DMDS) and or IPM practices might also be required and combined to achieve adequate control and economic crop productivity.
What the most current research has also told us is that all of the alternative fumigants with low vapor pressure and high boiling point are unable to distribute vertically below the traffic or tillage pan (and oftentimes horizontally to the bed shoulders) with current delivery and application methods originally developed for methyl bromide. Florida research has also demonstrated that plant-pathogenic nematodes are deeply distributed throughout the soil profile, and are oftentimes shielded from fumigants gases, which do not deeply penetrate the soil. This same research suggests that nematode control should be viewed as a composite of vertical management zones for nematode control and sustaining optimum crop production. The new approach targets treatments to deeper soil profiles where nematodes occur. The potential importance of the deeply distributed reservoir of nematodes and their effects on subsequent plant growth are now being considered within the testing phases of new deep shank and subsurface drip application technologies for soil fumigants. These new systems are expected to improve fumigant penetration into deep soil, overall nematode control, and crop yield response consistency. The benefits of deeper fumigant placement is expressed in reduced nematode damage potential to a given crop, which would have occurred from migrating individuals from soil depths where fumigants do not distribute. Based on these findings, it is believed that the root causes of yield and pest-control inconsistencies associated with the currently used fumigant nematicides have been identified, and new technologies that will help resolve these problems are under evaluation.
All of the fumigants are phytotoxic to plants and as a precautionary measure should be applied at least 3 weeks before crops are planted. When applications are made in the spring during periods of low soil temperature, these products can remain in the soil for an extended period, thus delaying planting or possibly causing phytotoxicity to a newly planted crop (Figure 20 and Figure 21). Field observations also suggest rainfall or irrigation which saturates the soil after treatment tends to retain phytotoxic residues for longer periods, particularly in deeper soil layers.


Summary
In summary, nematode control measures can be conveniently divided into 2 major categories including cultural and chemical control measures. None of these measures should be relied upon exclusively for nematode management. Rather, when practicality and economics permit, each management procedure should be considered for use in conjunction with all other available measures for nematode control and used in an integrated program of nematode management.
In addition to nematodes, many other pests can cause crop damage and yield losses that further enforce the development of an overall Integrated Pest Management (IPM) program, utilizing all available chemical and nonchemical means of reducing pest populations to subeconomic levels. An IPM approach further requires that growers attempt to monitor or scout fields for pest densities at critical periods of crop growth.
These crops share similar nematode problems and nematicide registrations, and are grown and handled similarly. Their most important nematode pests are root-knot (in sand, muck and Miami Dade County rock-based soils), stubby-root (in sand and muck soils), and sting (in sands) nematodes. Fumigants (Table 1) are much more consistently effective against root nematodes than the non-fumigants (Table 2); under some circumstances, non are more effective against stubby-root nematodes than are fumigants; most nematicides can be effective against sting nematodes if applied properly.
Tomatoes, peppers, and eggplant are produced on plastic-mulch-covered beds in many areas of Florida. These beds are routinely fumigated with a multi-purpose fumigant at the time they are covered for broad spectrum soil pest control. Several brands of the fumigants used most widely for tomatoes, including many different Telone/chloropicrin mixtures, have also been registered for peppers and/or eggplant. There is evidence to suggest that some formulations may be better suited for control of specific pest complexes (i.e., combinations of nematodes, weeds, and/or fungi). However, the grower must check the label of the product he is actually using to be sure that it is registered for the crops to be grown in the soil being treated. Most of the multi-purpose fumigants may be legally used to treat production fields for nearly any vegetable crop.
For an explanation of the tables in this document, see Nematode Management in Commercial Vegetable Production