This EDIS publication provides a description of the following pesticide characteristics: solubility, adsorption, persistence, and volatility.
Introduction
To understand how pesticides move in the environment, one must first understand certain physical and chemical characteristics of pesticides, as well as how these characteristics determine a pesticide's interaction with the environment. Some of these characteristics are described in the Physical and Chemical Properties section (Table 1) of a pesticide's Safety Data Sheet (SDS). Other pesticide properties are found in either the SDS' Stability and Reactivity section or Ecological Information section.
Solubility
Solubility is a measure of the ability of a pesticide to dissolve in a solvent, which is usually water. Pesticides that are highly soluble in water dissolve easily. Such pesticides are more likely to move with water in surface runoff or to move through the soil in water than less-soluble pesticides.
In the SDS, manufacturers use relative terms—such as miscible, dispersible, suspension, emulsifiable, and water solubility—to describe their product's solubility. Some manufacturers will use a numerical value for this description, such as 2.9 mg/L or ppm. Pesticides with a value of 100 ppm or less are considered relatively insoluble, while pesticides with values greater than 1,000 ppm are considered very soluble.
Adsorption
Adsorption is the process whereby a pesticide binds to soil colloids, which are microscopic inorganic and organic particles in the soil. Colloid is derived from the Greek term meaning glue-like. These particles have an extremely large surface area in proportion to a given volume. It has been calculated that 1 cubic inch of colloidal clay may have 200–500 square feet of particle surface area.
Adsorption occurs because of an attraction between the chemical and soil particles. Typically, oil-soluble pesticides are more attracted to clay particles and to organic matter in soil than water-soluble pesticides. Pesticide molecules with positive charges are more tightly adsorbed to negatively charged soil particles. A pesticide that adsorbs to soil particles is less likely to move from the application site than a chemical that does not adsorb tightly to the soil.
Persistence
Persistence is the ability of a pesticide to remain present and active in its original form during an extended period before degrading. A chemical's persistence is described in terms of its half-life, which is a comparative measure of the time needed for the chemical to degrade. The longer a pesticide's half-life, the more persistent the pesticide. Persistent pesticide residues are sometimes desirable because they provide long-term pest control and reduce the need for repeated applications. However, some persistent pesticides applied to soil, plants, lumber, and other surfaces or spilled into water or on soil can later harm sensitive plants or animals, including humans. It is especially important to prevent persistent pesticides from moving off-site through improper handling, application, drift, leaching, or runoff.
Application of persistent pesticides presents a hazard to persons and non-target animals entering a treated area and may lead to the presence of illegal residues on rotational food or feed crops. Check the label for statements about the persistence of the pesticide and for replanting restrictions. The rate of pesticide degradation relates to the persistence of the pesticide.
Degradation processes break down pesticide compounds into simpler and often less-toxic chemicals. Some pesticides break down rapidly—in a matter of days or even hours. Other pesticides can be detected in the environment for a year or more.
Pesticides are degraded by the following processes (Figure 1):
- Chemical degradation is the breakdown of chemicals by processes that do not involve living organisms, most commonly by hydrolysis, a reaction with water.
- Microbial degradation is the process in which chemicals are degraded by soil microorganisms, such as fungi and bacteria.
- Photodegradation is the breakdown of chemicals in reaction to sunlight.
Water and temperature both affect the degradation of pesticides. Warm, wet conditions can increase the speed of pesticide degradation; cool, dry conditions slow the degradation process.
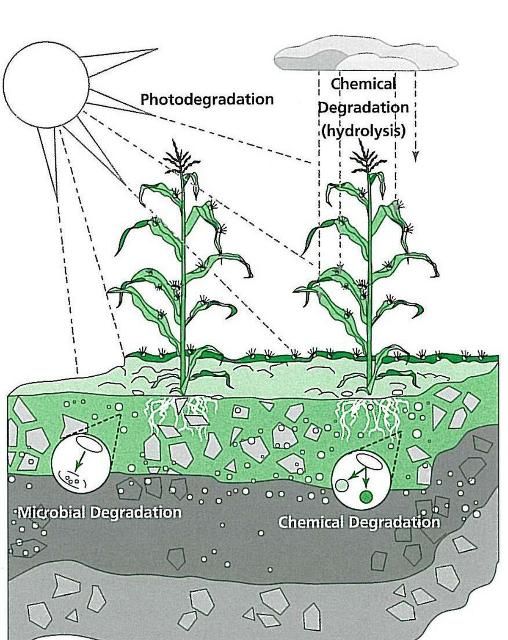
Credit: Crop Data Management Systems, CDMS
Volatility
Volatility is the tendency of a pesticide to turn into a gas or vapor. Some pesticides are more volatile than others. The likelihood of pesticide volatilization increases as temperatures and wind increase. Volatility is also more likely under conditions of low relative humidity.
The potential for a pesticide to volatilize is measured by its vapor pressure. This measurement may be described in units of Pa (Pascals) or mmHg (millimeters of mercury). Pesticides that have high vapor-pressure values are more volatile. Vapors from such pesticides can move off-site and cause injury to susceptible plants. Some volatile pesticide products carry label statements that warn handlers of the product's potential for vapor movement (Figure 2).

Credit: National Pesticide Applicator Certification Core Manual