This document is an abbreviated look at the botany of the sugarcane plant. Gross anatomy and a few of the major physiological processes of the plant are discussed.
Main Parts of the Plant
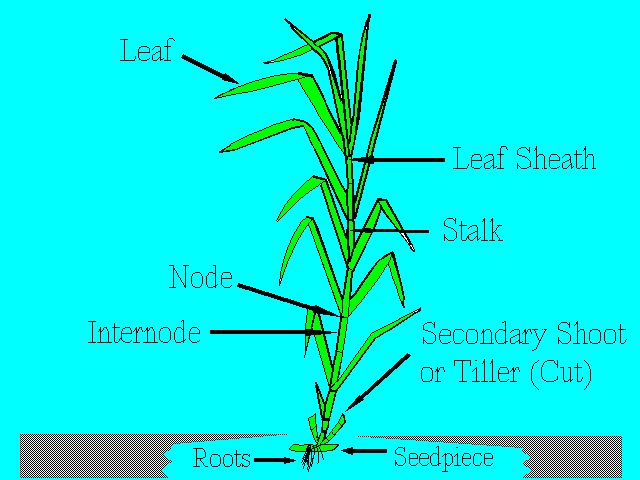
The main parts of the sugarcane plant are the stalk, leaf, and root system. Figure 1 shows these parts, and others, which are examined in more detail below.
The Stalk
The stalk consists of segments called joints. Each joint is made up of a node and an internode (Figure 1 and Figure 2). The node is where the leaf attaches to the stalk and where the buds and root primordia are found. A leaf scar can be found at the node when the leaf drops off the plant. The length and diameter of the joints vary widely with different varieties and growing conditions. In general, however, the joints at the base and the top are short with short internodal length and with long internodes in the middle of the stalk.
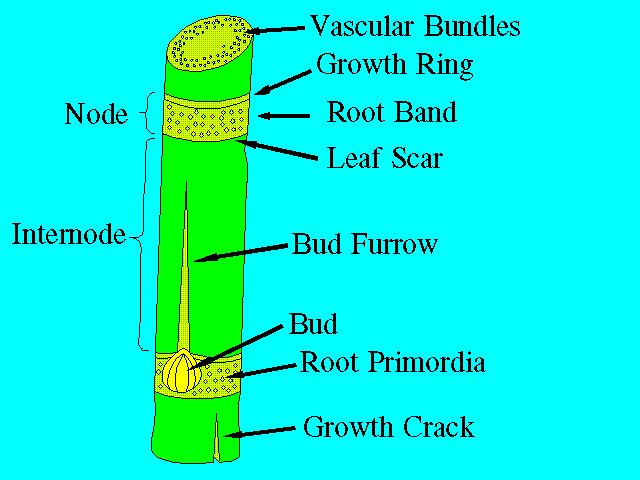
The buds, located in the root band of the node, are embryonic shoots consisting of a miniature stalk with small leaves (Figure 2). The outer small leaves are in the form of scales. The outermost bud scale has the form of a hood. Normally, one bud is present on each node, and they alternate between one side of the stalk to the other. Variations in size, shape, and other characteristics of the bud provide a means of distinguishing between varieties. The root band also contains loosely defined rows of root primordia. Each primordium exhibits a dark center, which is a root cap, and a light colored "halo."
When seed-cane is planted, each bud may form a primary shoot. From this shoot, secondary shoots called tillers may form from the underground buds on the primary shoot. In turn, additional tillers may form from the underground secondary shoot buds.
The colors of the stalk seen at the internodes depend on the cane variety and environmental conditions. For example, exposure of the internodes to the sun may result in a complete change of color. The same variety grown in different climates may exhibit different colors. All colors of the stalk derive from two basic pigments: the red color of anthocyanin and the green of chlorophyll. The ratio of the concentration of these two pigments produce colors from green to purple-red to red to almost black. Yellow stalks indicate a relative lack of these pigments.
The surface of the internode, with the exception of the growth ring, is more or less covered by wax. The amount of wax is variety-dependent. Where the internode is exposed to the elements, a black mold usually develops on the waxy surface.
The top of the stalk is relatively low in sucrose and, therefore, is of little value to the mill early in the harvest season. However, the top 1/3 contains many buds and a good supply of moisture and nutrients which makes it valuable as seed cane for planting.
A cross section of an internode shows, from the outside to the center, the following tissues: epidermis, cortex or rind, and ground tissue with embedded vascular bundles.
The epidermis is a single superficial layer of cells that exhibit different patterns that are variety dependent. Generally, the patterns are formed by two cell types, the so-called long cells and short cells, that alternate with one another.
The cortex or rind consists of several layers of cells just inside the epidermis. The cells of the rind are thick-walled and lignified. These cells help strengthen the stalk.
More toward the center, the ground tissue contains the vascular bundles with the xylem and phloem. Xylem tissue conducts water and its dissolved minerals upward from the roots, and phloem conductive tissue transports plant-manufactured nutrients and products, for the most part, downward toward the roots. The vascular bundles are much smaller and more prevalent toward the periphery of the stalk.
Two types of cracks are sometimes found on the surface of the stalk: harmless, small corky cracks that are restricted to the epidermis, and growth cracks that may be deep and run the whole length of the internode. Growth cracks are harmful since they allow increased water loss and expose the stalk to disease organisms and insects. Growth cracks are dependent on variety and growing conditions.
The Leaf
The leaf of the sugarcane plant is divided into two parts: sheath and blade, separated by a blade joint (Figure 3). The sheath, as its name implies, completely sheaths the stalk, extending over at least one complete internode. The leaves are usually attached alternately to the nodes, thus forming two ranks on opposite sides. The mature sugarcane plant has an average total upper leaf surface of about 0.5 square meters, and the number of green leaves per stalk is around ten, depending on variety and growing conditions.
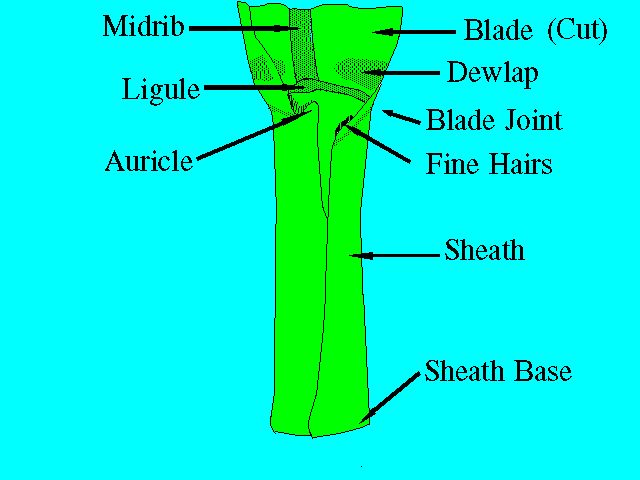
A cross-section through the leaf blade would show three principal tissues: (a) epidermis, (b) mesophyll, and (c) veins or fibrovascular bundles. The epidermal cells protect the underlying tissues from injury and drying. The epidermis contains stomata for controlling the exchange of gases. The mesophyll, or middle leaf tissue, contains the cells that perform most of the photosynthesis. The fibrovascular bundles contain the xylem and phloem elements, which conduct water and nutrients to and from the leaves. In addition to the above, there can be found other fibrous tissue for aiding in shaping and mechanically strengthening the leaves.
The blade joint is where two wedge-shaped areas called dewlaps are found (Figure 3). The color, size, and shape of the dewlaps on a mature plant are more or less characteristic of a variety. The top visible dewlap leaf is a diagnostic tissue that is frequently used in nutritional studies. For more information, see UF/IFAS EDIS publication SS-AGR-259 Sugarcane Leaf Tissue Sample Preparation for Diagnostic Analysis (https://edis.ifas.ufl.edu/SC076).
The leaf sheath is similar in structure and function to the leaf blade. It is slightly simpler, however, in that it lacks some of the more specialized cells of the leaf blade.
The ligule is a membranous appendage inside of the sheath that separates the sheath from the leaf blade (Figure 3). It is a slightly asymmetric organ whose color, size, and shape are age- and variety-dependent.
The auricles, as their name implies, are ear-shaped appendages located at the upper part of the sheath margin. Not all sheath margins have auricles.
Leaf pubescence, or the covering of the various leaf parts with short hairs, is also variety and age dependent. Pubescence is not found on the leaf blade of commercial varieties, but does exist in sugarcane germplasm. Sheath pubescence can be used to identify plants.
The Root System
The function of the root system is twofold: first, it enables the intake of water and nutrients from the soil; and second, it serves to anchor the plant. Two kinds of roots will develop from a planted seed piece. The set roots, which arise from the root band, are thin and highly branched; the shoot roots, originating from the lower root bands of the shoots, are thick, fleshy, and less branched (Figure 4).
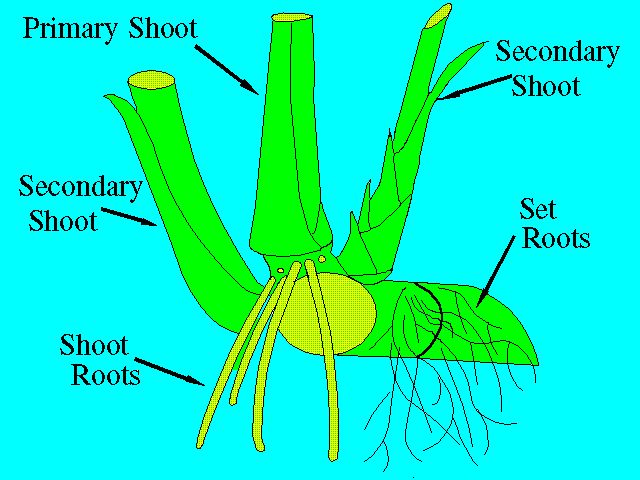
Before shoots form, the germinating seed piece must depend entirely on the set roots for water and nutrients. The set roots, however, are only temporary, and their function will eventually be taken over by the shoot roots as they develop. The life of the shoot root is also limited. Each new tiller (shoot) will develop its own roots that eventually take over the function of the original shoot roots. This rejuvenation, governed by the periodicity of tillering, is important because it allows the plant to adjust to changing environmental conditions.
A longitudinal section of a root tip consists mainly of four parts: the root cap, the growing point, the region of elongation, and the region of root hairs. The root cap protects the tender tissues of the growing point as the root pushes through the soil. The growing point consists mainly of an apical meristem, where cell division takes place. In the region of elongation, the cells increase in size and diameter until they reach their ultimate size. The region of root hairs is characterized by epidermal cells forming outgrowths (hairs) that dramatically increase the roots absorbing surface.
The Inflorescence
When a sugarcane plant has reached a relatively mature stage of development, its growing point may, under certain photoperiod and soil moisture conditions, change from the vegetative to reproductive stage. This means the growing point ceases forming leaf primordia and starts the production of an inflorescence. The inflorescence, or tassle, of sugarcane is an open-branched panicle. Each tassle consists of several thousand tiny flowers, each capable of producing one seed. The seeds are extremely small and weigh approximately 250 per gram or 113,500 per pound.
For commercial sugarcane production, inflorescence development is of little economic importance in Florida. Generally, a day length close to 12.5 hours and night temperatures between 68 and 77 degrees F will induce floral initiation. Temperatures that are too low and/or water stress inhibit inflorescence development. Nighttime temperatures are generally too low to produce viable sugarcane seed in Florida.
Germination
Commercial sugarcane is propagated by cuttings of the stalk (seed cane) containing usually two or more nodes with buds. The bud, a miniature stalk with its growing point and root and leaf primordia, forms the new shoot. In addition, a seed piece contains root primordia within its root band, which develop into set roots which function until the young shoot develops its own roots.
The transition from the dormant into the active stage constitutes a complex phenomenon characterized by changes in the activity of enzymes and growth regulating substances (hormones, auxins). Maximum germination and shoot vigor will result when both internal and external factors are optimal. Fortunately, these factors can be regulated to a considerable degree by cultural methods.
Tillering
Tillering, or development of secondary shoots, is a beneficial characteristic of a variety because it provides the plants with the appropriate number of stalks for a good yield. Also, tillering increases the rate of canopy closure which aids in weed control. Varieties differ greatly in their tillering capability and the ultimate number of tillers present at harvest.
Besides variety differences, numerous other factors influence tillering. Ultimately, tillering is related to the phenomenon of "apical dominance" and, therefore, plant hormones are involved in the process of tillering. The most important external factors influencing tillering are light, temperature, nutrition, moisture and the spacing of the plantings. Of these factors, experiments have shown that light is the most significant. Increasing light intensity and duration, in general, greatly increases tillering.
In young cane fields the period of profuse initial tillering is followed by a wave of mortality as soon as the rows close in. More than 50% of the number of the initial stalks may die. Much of the mortality is due to light competition.
Growth
The growth of the cane plant does not proceed at a uniform rate. Development starts slowly in the germinating bud, and it increases gradually until a maximum is reached, which is followed by a gradual decrease (Figure 5). The time period in which the plant is growing rapidly is called the "grand growth period."
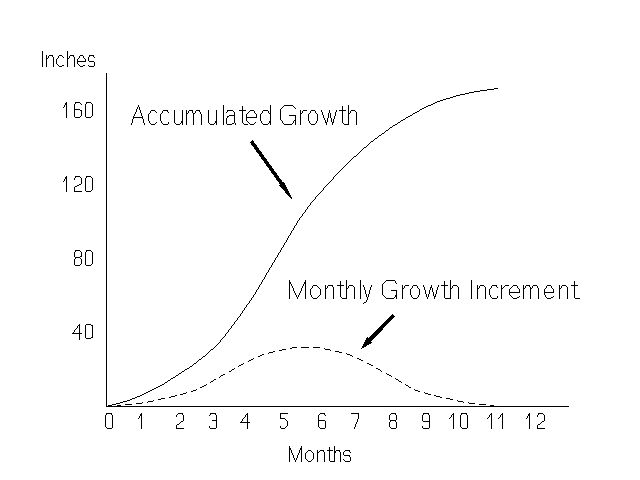
Cane growth is governed by a complex of internal and external factors. Not surprisingly, the more important external factors affecting growth are moisture, temperature, light, soil condition and nutrition. It is beyond the scope of this fact sheet to deal with all these individual factors. For further information, see the UF/IFAS EDIS topic page at https://edis.ifas.ufl.edu/TOPIC_Sugarcane_Cultural_Practices.
Nutrition
The essential elements for a healthy sugarcane crop include carbon, hydrogen, oxygen, nitrogen, phosphorus, potassium, calcium, magnesium, boron, chlorine, copper, iron, manganese, molybdenum, sulfur, and zinc. Silicon, although not strictly needed for the sugarcane plant to complete its life cycle, may enhance sugarcane production significantly. An over-abundance of one element may cause a deficiency or toxicity of another. Hence, there is a need for a good nutritional balance to produce the healthiest plants. Since relatively large quantities of N, P, K, S, Mg, and Ca are needed by the plants, these are referred to as macronutrients. The remainder of the elements are usually called micronutrients.
Although nitrogen constitutes only a fraction of one per cent of the total dry matter of a mature sugarcane plant, it plays a role as important as C, H, and O, which together form more than 90 percent of the dry matter. Nitrogen deficiency is common in sandy soils and it is uncommon in organic soils.
Nitrogen has the greatest influence on cane ripening (discussed below) of all the nutrient elements. Sugarcane will store a higher percent of sucrose when nitrogen is limited 6 to 8 weeks prior to harvest.
Sugarcane nutrition is an extensive topic. Refer to UF/IFAS EDIS fact sheet SS-AGR-228 Nutritional Requirements for Florida Sugarcane (https://edis.ifas.ufl.edu/SC028) for more information.
Energy Relations
Photosynthesis
Photosynthesis is the process by which plants transform the radiant energy of the sun into chemical energy stored in carbohydrates. Photosynthesis involves a fairly long series of complicated reactions, but can be summed up in the following equation:
6CO2 + 6H2O (+ Sunlight) ----> C6H12O6 + 6O2
Sucrose (C12H22O11 ) is the major carbohydrate formed, used, and stored by the sugarcane plant.
Respiration
Respiration is the process in which the stored chemical energy is released to the plant for its various functions such as growth and moving certain nutrients against concentration gradients. Chemically, in essence, it is the reverse of photosynthesis:
C6H12O6 + 6O2 ----> 6CO2 + 6H2O (+ Usable Energy)
The balance of the carbohydrates not used for the building of the plant frame or the production of energy is stored in the stalk mainly in the form of sucrose.
Translocation
The rapid translocation of sucrose and other carbohydrates and nutrients takes place mainly in the phloem. These plant foods move to the tissues utilizing them. Thus, food can move from the leaves where manufactured, to the stalk, regions of growth, and roots. The loading and unloading of the phloem elements could be by diffusion in the direction of a concentration gradient, but is more likely to be a metabolically-mediated, regulated, and reversible process.
Ripening
The storage of sucrose in the stalk is known as ripening. Ripening is a joint to joint process, and the degree of maturity of the individual joints depends on their age. In young plants, the sucrose content exhibits a distinct maximum that is located approximately at soil level. The sucrose content in these plants decreases through the stalk toward the top of the stalk. As the plant matures, a more uniform sucrose content is found throughout the stalk except for the top few internodes and the below-ground stool.
Certain plant growth regulators can accelerate the accumulation of sucrose in the stalk. These regulators, or chemical ripeners, can be used to a commercial advantage. See UF/IFAS EDIS fact sheet SS-AGR-215 Sugarcane Ripeners in Florida Sugarcane (https://edis.ifas.ufl.edu/SC015) for more information on this topic.
Evapotranspiration
Transpiration is defined as the giving off of water vapor by the plant, almost exclusively from the leaves. Evapotranspiration is the release of water vapor from a unit surface of land which includes the soil and the plants. The plant controls the inflow and outflow of water vapor and other gases by way of stomata on the leaf surface. The stomata can be opened or closed by a pair of guard cells that respond to leaf water status.
Experiments with Florida sugarcane have shown it requires about 220 to 275 lb of water to produce one lb of dry biomass, and about 520 to 680 lb of water to produce one lb of sugar.
The sugarcane plant's response to drought is somewhat variety-dependent. The main line of defense may be tight control of stomatal opening and closing. Other mechanisms include leaf canopy collapse and leaf senescence that can reverse itself once the drought ends.
Summary
Sugarcane botany is similar to other grasses, with the major exception that the harvested economic product is sucrose stored in the stalk rather than grain or fodder. The long growth duration of sugarcane and its tolerance to fluctuating water tables mimic the native sawgrass vegetation and make sugarcane particularly well-suited for the Everglades Agricultural Area of Florida.