What is Cryptobia?
Cryptobia species are flagellated protists, distantly related to Hexamita and Spironucleus, but not nearly as well understood. Like Hexamita and Spironucleus, Cryptobia spp. are very tiny (single-celled) organism and, consequently, can be difficult to identify and study. There have been 52 species of Cryptobia identified in fish; however, because of the parasites' small size and difficult taxonomy, these may not all be separate species. Of the 52 species that have been identified, five are classified as ectoparasites that infect the gills and skin; seven are classified as enteric parasites that infect the gastrointestinal system; and 40 are classified as hemoflagellates which are found in the bloodstream. It has been proposed that the hemoflagellates be assigned to a subgenus called Trypanoplasma. The hemoflagellates have an indirect life cycle and are transmitted by leeches, whereas the gastrointestinal and ectoparasitic forms have direct life cycles.
Cryptobia iubilans and Cichlids
Cryptobia iubilans was first recognized in cichlids around 40 years ago. The organism is typically associated with granulomas (a tissue reaction) in the stomach, but systemic infections that involve the organism in blood and organ systems (including liver, gall bladder, kidney, ovary, brain, and eye) have been reported. It is not known how the organism is able to spread from the intestinal tract to other organs, or what causes the internal spread. Mortalities associated with the systemic form may exceed 50% of the infected population.
The gastrointestinal form of Cryptobia has been reported in East African and Central American cichlids, including: Herichththys cyanoguttatus, Cichlasoma meeki, Cichlasoma nigrofasciatum, and Cichlasoma octofasciatum. We have found it in many additional species, but more detailed work has been done in Pseudotropheus zebra, Symphysodon spp., Aulonocara jacobfreibergi, Placidochromis milomo, and Astronotus ocellatus. It appears to be uncommon or exceedingly rare in angelfish (Pterophyllum spp.).
In the summer of 1995, there was an outbreak of the systemic form of Cryptobia iubilans in cichlids at the Chicago Shedd Aquarium. The outbreak resulted in loss of 50% of the collection of East African cichlids including Cyphotilapia frontosa, Dimidiochromis compressiceps, and Aulonocara stuartgranti. The outbreak seemed to originate with the Aulonocara that had been purchased from a midwest wholesaler. While the fish were in quarantine, the infection spread to Cichlasoma meeki and C. nicaraguense housed in the same tank. From there it spread to the C. frontosa and D. compressiceps that were housed in separate tanks but shared the same water due to a common filtration system. The sick fish went off feed for one to two days, becoming progressively more listless and withdrawing from contact with other fish. Just prior to death, they would move to the surface of the water and their respiration rate would increase dramatically, suggesting that they were hypoxic (suffering from low dissolved oxygen). Closer examination of fish at this stage of the disease revealed severe anemia, with packed cell volumes around 5% (normal should be greater than 30%). Death usually occurred within 24 hours of the development of severe anemia.
Veterinarians at the Shedd Aquarium wanted to see how many species in their collection carried the parasite so they sacrificed 60 apparently healthy fish, and found evidence of Cryptobia iubilans in all but one (98% prevalence). These fish had granulomatous gastritis (the tissue reaction in the stomach) but no evidence of the systemic disease. The affected species included Haplochromis macula, Cichlasoma nicaraguense, Labeotropheus fuelleborni, Cichlasoma aureus, Pseudotropheus zebra and P. elongatus. Since the 1995 epizootic (disease outbreak), Shedd Aquarium has instituted a new quarantine protocol for all cichlids. All incoming cichlids are subjected to a minimum 60-day mandatory quarantine. A number of animals are screened for the presence of Cryptobia; any infected cichlid is culled, a number of animals are screened for the presence of Cryptobia and the presence of the live parasite will then require treatment (see "management").
After many years of diagnostics at the University of Florida and at other laboratories around the country, it appears that Cryptobia iubilans is not uncommon among cichlids, and that environmental and other factors determine the extent of disease.
Comparing Cryptobia and Spironucleus Infections
Clinical Disease
Both Cryptobia and Spironucleus can result in similar disease scenarios on cichlid farms. Both parasites become more serious under conditions of crowding, poor sanitation, high organic load, and handling stress. Diet also may play a role in the development of the disease. It has been demonstrated in laboratory mice that changes in the intestinal bacterial flora, caused by changes in diet, can affect the presence of intestinal flagellates, suggesting greater potential for clinical disease.
Enteric disease from either parasite may result in low level chronic mortality, "wasting" or poor growth. The effect of Spironucleus is more serious in fry and very young fish. It is not known if this is also true for Cryptobia, but there is some evidence that supports this belief. The impact of either disease on reproduction is not well understood; however, we believe that breeders heavily infected with Spironucleus produce poor quality eggs and weak fry.
Diagnosis
Spironucleus can be tentatively identified by observing the motile trophozoites in smears of intestinal contents or feces. Spironucleus infestations do not result in granuloma formation. Identifying the parasite to genus requires both transmission and scanning electron microscopy and therefore cannot be done on a routine basis. Identification of granulomas in thin wet mounts of stomach tissue (Figure 1) and, rarely, motile flagellates in stomach fluids or tissues, strongly suggest disease caused by a Cryptobia iubilans infection. Because these granulomas are indistinguishable from the granulomas observed with Mycobacterium, an acid-fast stain (eg. Ziehl-Nielson) should be used to rule out that important disease (see UF/IFAS Extension Fact Sheet No. VM-96). In most instances, motile forms of Cryptobia will not be seen on wet mounts that are examined with a light microscope. Electron microscopy is also required to confirm the identity of this organism.
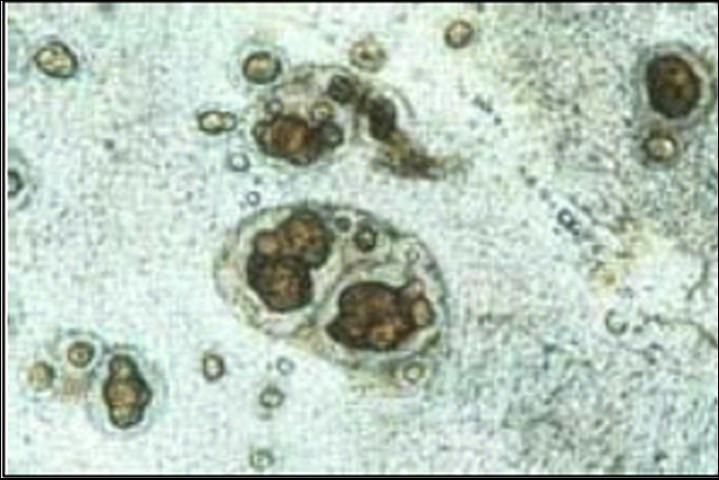
Transmission
Both Spironucleus and Cryptobia have direct life cycles. Infective forms are shed with feces, and ingestion of these forms or ingestion of organs from other infected fish (i.e., cannibalism) are thought to result in infection. Both organisms can live in the water column for at least a few hours. Always remove carcasses as quickly as possible when they are found, since both parasites may be spread by ingestion of infected tissue.
Management
Because environmental factors (poor water quality, crowding, or poor diet) can facilitate disease in fish, management for both Spironucleus and Cryptobia iubilans should include improving environmental conditions where needed.
Spironucleus usually responds well to metronidazole administered in feed or as a bath. The recommended dose in feed is 1% (4.5 grams active drug per pound of feed) fed daily for five consecutive days (see UF/IFAS Extension Fact Sheet No. VM-67). The bath treatment is 6 mg/L (250 mg added to 10 gallons of water), followed by a water change four to eight hours after treatment, repeated daily for five days (see UF/IFAS Extension Fact Sheet No. VM-67). These regimes have been very effective for control of Spironucleus in cichlids for the past twenty years.
Treatment for a Cryptobia iubilans infestation is considered more difficult, and current recommendations are based on laboratory experimental trials. Part of the difficulty may be that the parasite seems to have an intracellular stage. Parasites are occasionally seen in phagocytic cells, called macrophages, which are part of the immune system and are supposed to destroy foreign materials by engulfing them. Cryptobia seems to be able to live within these cells rather than being destroyed by them. This can make it difficult to treat Cryptobia because most drugs are not able to penetrate the cell wall of a macrophage. Experiments run at the Tropical Aquaculture Laboratory suggest that these two treatment regimens may decrease the infection load: a) a bath treatment with dimetridazole (80 mg/L for 24 hrs, followed by a 80–100% water change, repeated daily for 3 days); or b) a bath treatment with 2-amino-5-nitrothiazol (10 mg/L for 24 hrs, followed by a 80-100% water change, repeated daily for 3 days).
Summary
Cryptobia iubilans is not a new parasite of cichlids but has received significant attention in the past few years. Cryptobia iubilans infestations may be confused by some with spironucleus, which is much easier to control than cryptobia iubilans. It seems to be widespread in East African cichlids and has been found in Pseudotropheus zebra immediately following importation from Lake Malawi, suggesting that it occurs naturally in wild fish. It has also been found in some South American cichlids, most notably, discus. The parasite usually causes a granulomatous gastritis and may be associated with chronic low-level mortality. A systemic form of the disease has been reported in captive East African and Central American cichlids. This form was associated with acute mortalities and loss of 50% of affected animals. Experimental treatments that may provide some positive results include dimetridazole or 2-amino-5-nitrothiazol, but correcting any management problems is critical to reducing losses. Water quality, stocking density and diet may all effect the severity of infection. More work is needed to learn more about this parasite and its control.
Additional Reading
Yanong, R.P.E., E. Curtis, R. Russo, R. Francis-Floyd, R. Klinger, I. Berzins, K. Kelley, and S.L. Poynton. 2004. Cryptobia iubilans infection in juvenile discus. Journal of the American Veterinary Medical Association 224 (10): 1644–1650.