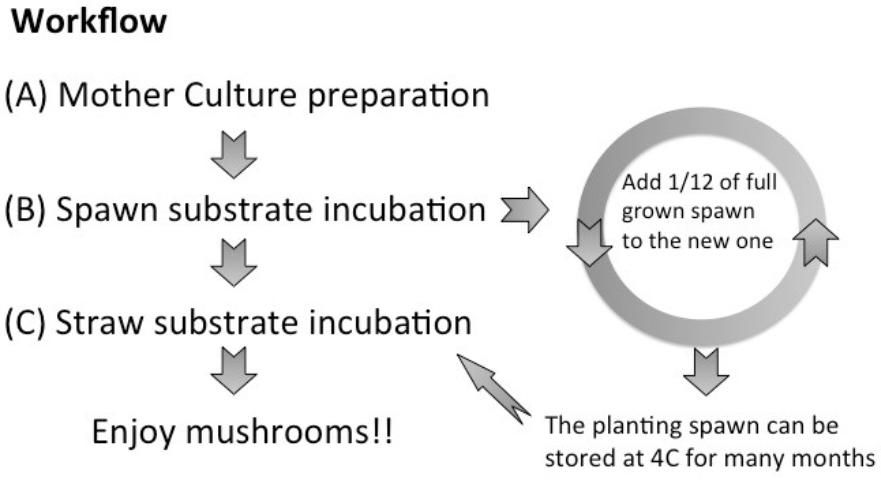
This publication details the cultivation of oyster mushrooms from mother culture isolation (Figure 1A) to spawn preparation (Figure 1B). This protocol can be used by both homeowners and commercial cultivators. The workflow for oyster mushroom growing is summarized in Figure 1. The process for lignocellulose substrate inoculation and spawn run (Figure 1C) are described in another EDIS article, "D.I.Y. FunGuide: Grow Your Own Oyster Mushrooms at Home" (https://edis.ifas.ufl.edu/publication/ss662).
Oyster mushrooms can be cloned (isolated into pure culture) from the basidiocarp onto a prepared medium. On the surface of a medium, the fungal mycelium can grow out from the basidiocarp tissue and be used as the mother culture inoculum to prepare spawn. The growing mycelium can be periodically sub-cultured onto a fresh medium. The stored strain can be maintained stably on media or as spawn stocks at 4°C for non-tropical Pleurotus strains. Careful culture storage is vital is to ensure that the specific strain of oyster mushroom is maintained and available for spawn production. The spawn is made by inoculating grain with mycelium from pure cultures. Although spawn-making is an adventurous, demanding task, it can be feasible with careful methods.
Knowing the Species of Mushroom that You are About to Cultivate
For mushroom cloning (isolation), it is recommended to purchase oyster mushrooms from the market to be sure it is an edible species and strain suitable for cultivation. Learning the basic characteristics of the oyster mushrooms is important to verify the cultivated species. Oyster mushroom (Pleurotus spp.) is a gilled mushroom. The most common species of Pleurotus include pearl oyster mushroom (P. ostreatus), aspen oyster mushroom (P. populinus), and phoenix oyster mushroom (P. pulmonarius); are all choice edibles (Petersen and Krisai-Greilhuber 1996; Stamets 2000; Vilgalys 1997). Generally, species of Pleurotus are edible. However, there are potentially dangerous lookalikes in other fungal genera. One example is Pleurocybella (Pleurotus) porrigens (see Appendix A, messiah.edu). Appendix A provides the references and web resources to morphologically differentiate the oyster mushrooms from poisonous and undesirable lookalike mushroom species.
Basic Materials
Spawn production requires a microbiological laboratory-style workspace, characterized by the following:
For oyster mushroom mother culture (fungal pure culture):
- Petri plates or small glass jars, such as baby food jars.
- Agar medium such as potato dextrose agar (PDA) medium or malt extract agar (MEA) medium.
- Tweezers, needles, and spatulas.
- Sterilization flame of alcohol lamp or propane burner.
- Parafilm or tape.
- Ethanol (200 proof alcohol) for burning and preparation of 70% alcohol as disinfectant.
For spawn preparation:
- Plant seeds, such as millet, wheat, sorghum, and rye. Note: the use of a specific seed type depends on availability, cost, and preference.
- Mason glass jars (1 L) with metal screw caps. There should be a small (2–2.5 cm) hole created in the center of the lid and guarded with a Millipore filter or cotton plug.
- Ethanol (200 proof alcohol) for burning and preparation of 70% alcohol as disinfectant.
Mother Culture Preparation
Prepare Media Plates
Prior to attempting mother culture isolation or transfer, one needs to have prepared media plates for pure culturing. Petri plates or small glass jars such as baby food jars can be used as the media containers. Two growth media, one agar-based and one gelatin-based, can be used to culture the mycelium of oyster mushroom; they are listed below.
- Potato dextrose agar (PDA)
- 200 g of thin slices of potato.
- 20 g glucose, or 20 g sucrose (table sugar).
- 15 g of agar powder.
- Boil the potato slices in 500 ml of water until the potato tissue is completely cooked and soft. Strain the 500 ml of potato broth through several layers of cheesecloth.
- Add the sugar plus the agar into 500 ml of water. Boil for about 5 min to completely dissolve agar and sugar and keep stirring to avoid burning the material (fungi don't like burned food).
- Combine the 500 ml potato broth plus the 500 ml of dissolved agar and sugar to get 1,000 ml of PDA (bring up to a 1000 ml volume with additional water if need be), which is ready for sterilization using an autoclave. Alternatively, a pressure cooker may be used to sterilize the medium. In general, 30 minutes at 15 psi is suggested for the sterilization. Be certain to follow the pressure cooker manufacturer guidelines for safety. Let the autoclave or the pressure cooker cool down according to equipment instructions before opening. Do not open when there is positive pressure inside; otherwise, the media may boil over. Let the sterilized media cool down to about 50°C (122°F) before pouring into plates or suitable containers, Petri plates, or previously sterilized small glass jars or containers. Alternatively, when using small jars, the media could be placed inside the jars before autoclaving. Hence, the user will avoid the pouring phase, as the containers with media will be ready for use after sterilization. The pouring step should be performed under aseptic conditions, and cooled down and solidified under the same conditions. Once inoculated, agar plates used for fungal growth should be sealed by parafilm or tape to prevent possible contamination or getting accidentally opened. If jars are used, they should have metal caps that tightly close their opening. Observe the mother culture plate for cleanness and absence of contamination with bacteria and/or other unwanted fungi. Maintain clean cultures by discarding contaminated plates.
2. Gelatin medium
The gelatin medium can be also used to make media to support the growth of oyster mushroom mother cultures (Figure 2), although Oyster mushroom fungi (Pleurotus spp.) do not grow as well on gelatin medium as they do on PDA medium. It is relatively easy to purchase in public or online stores and make gelatin medium compared to PDA. Below is the protocol for gelatin medium preparation.
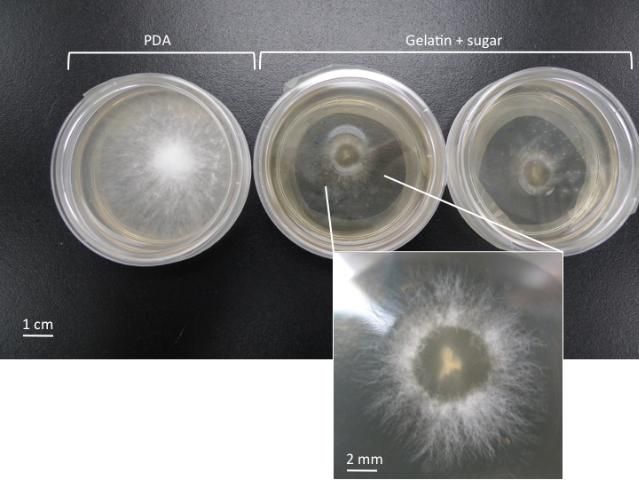
Credit: Chih-Ming Hsu
- Dissolve 2 g of sugar mixed with 18 g of gelatin powder (Knox) in 125 ml water. Boil for about 5–10 min to completely dissolve the gelatin-sugar mix and keep stirring to avoid burning the material. If available, using the pressure cooker for this step can reduce the contamination rate. Close the pot lid. Let it cool down to about 50°C (122°F) before pouring into plates or suitable containers as described above for PDA medium.
Tissue Culture Isolation for Mother Culture Production
Pure cultures of oyster mushroom can be obtained by tissue culture from desired mushroom fruiting bodies, the basidiocarps. The inside tissue of a fruiting body is exposed by pulling apart the mushroom cap to expose the inside uncontaminated tissues (Figure 3). Then, pluck tiny pieces of the inside tissue using sterile fine-tip tweezers and transfer onto the agar or gelatin media. Ideally, this is done inside a still-air transfer chamber or a laminar flow hood to avoid airborne contamination. Observe for growth of new hyphae, and when it becomes evident growing out of the transferred mushroom pieces without any contamination, then the new isolate is sub-cultured onto new growth medium under aseptic conditions. New cultures are assigned unique numbers or codes and maintained as mother cultures (pure cultures). Store the obtained mother cultures at 4°C (39°F) and sub-culture every 6–12 months. However, if a gelatin medium is used for mother cultures, the long-term storage is not recommended. The gelatin medium culture may turn soft or be liquidized in few weeks after storage.
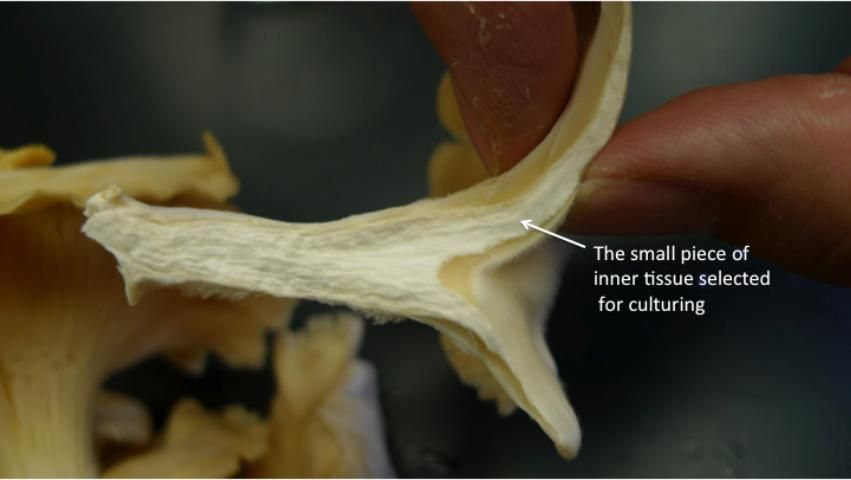
Credit: Chih-Ming Hsu
Making Your Own Spawn
Spawn Preparation and Inoculation: Soaking and Sterilization of the Seeds' Spawn Substrate
Millet seed is an excellent substrate for producing oyster mushroom spawn. Seeds should be soaked for about twelve hours (Figure 4). Longer soaking causes seed fermentation, lowering seed quality and its suitability for spawn preparation. After draining, transfer the soaked seeds into containers such as the Mason jars or the commercial or homemade spawn bags. Sterilize the seeds inside those containers by heat and vapor pressure sterilization using a pressure cooker (Figure 5). In general, 30 minutes at 15 psi is suggested for the sterilization. Be certain to follow the pressure cooker manufacturer guidelines for safety. Optionally, though it may not sufficiently reduce microbes on the seeds, seeds can also be prepared by boiling with a small amount of gypsum added during the boiling process. In this case, boil the seeds for at least 60 min. All kinds of glass containers can be used, such as narrow neck bottles and wide mouths jars. In all cases, provision must be made to allow gas exchange for the growing mycelium inside the container and be secure against contamination. Wide-mouth glass jars with a hole driven through their metal lid and plugged with cotton for gas exchange are a good choice for spawn preparation.

Credit: Khalid Hammeed

Credit: Khalid Hammeed
It is necessary to shake the seeds inside the container soon after it is out of the pressure cooker in order to mix the top portion of those seeds (which tend to be drier than the ones sitting in the bottom of the container where moisture collects during cooling) and to prevent agglutination of the seeds into one big mass.
The sterilized seeds, which are the substrate for the oyster mushroom culture to grow on to produce spawn, should be inoculated with mycelium from a pure mother culture under aseptic conditions to prevent contamination and produce clean, healthy spawn. The aseptic, germ-free condition can be created by working under a laminar flow hood or inside an airtight transfer room with strict measures of keeping out all sources of contamination. For the home-based working environment where obtaining these conditions is difficult, having a clean work environment is the key to success. For example: (1) use 70% of ethanol to clean the working table prior to work on inoculation; (2) use a flame to sterilize transfer tools taking appropriate safety measures; (3) make the preparation/inoculation process as fast as possible without been interrupted; (4) work within a still-air transfer box.
Inoculation of the Spawn Substrate (Seeds)
The metal transfer tool needs to be sterilized immediately before each transfer with their tip heated red-hot in a flame from an alcohol lamp or a burner. It is highly recommended that operators should wear gloves and manipulate the culture transfer and inoculation deep inside a transfer hood.
Use clean, healthy growing cultures that have just filled the surface of a small Petri plate (60 mm diameter) or 1/4 of a large plate (100 mm diameter) to inoculate 400–450 g substrate.
The inoculum (our mother culture) in this case is a healthy, non-contaminated, actively growing culture of the desired strain growing on agar or gelatin (Figure 6). Use a wide blade surgical scalpel #12 or equivalent tool to cut the agar culture plate. Heat, sterilize, and then cool down the blade before cutting the agar culture plate. Cut the fungal mycelium and the agar into small cubes. Aseptically transfer those cubes on to the previously sterilized seeds inside the spawn container. Shake the inoculated spawn container to get the culture pieces distributed inside the substrate, establish growth centers inside, and promote rapid colonization of the substrate (Figure 7). Incubate the inoculated spawn containers at 23°C (73°F) in darkness. A clean cardboard box can be used as an incubation container at a room temperature of 23–25°C. The spawn containers can also be protected from light by wrapping with aluminum foil (Figure 8). The incubation period lasts until complete colonization of the substrate has occurred (around 10 to 20 days) (Figure 9). The mature spawn should then be stored in darkness in a refrigerator at 4°C (39°F) until used; however, cultures of tropical strains of Pleurotus should not be refrigerated. The stored spawn can last for many months unless it dries out or gets contaminated.

Credit: Khalid Hameed

Credit: Khalid Hameed

Credit: Khalid Hameed

Credit: Khalid Hameed
Using the First Batch of Spawn as "Master Spawn" for Increasing the Spawn for use as "Planting Spawn"
In this case, the first batch of prepared spawn is used as inoculum for a second set of containers with sterilized seeds to bulk up the amount of spawn. Then, the first preparation is called "master spawn" and the subsequent preparation called "planting spawn," which is then used to inoculate straw to produce oyster mushrooms (Figure 1).
Appendix A
Examples of resources for the identification of oyster mushrooms and their lookalikes. It is suggested to follow the suggestion in the Field guide to common macrofungi (General Technical Report NRS-79): “DO NOT eat any mushroom unless you are absolutely certain of its identity.”
- Ostry ME, Anderson NA, O’Brien JG. 2010. USDA Field Guide to Common Macrofungi in Estern Forests and Their Ecosystem Functions (General Technical Report NRS-79, page 13): https://www.fs.usda.gov/nrs/pubs/gtr/gtr_nrs79r.pdf
- Midwestmycology.org: http://www.midwestmycology.org/Mushrooms/Species%20listed/Pleurotus%20species.html
- Mushroom-collecting.com: http://mushroom-collecting.com/
- Kuo, M. 2009. "Pleurotus pulmonarius: The summer oyster." Retrieved from http://www.mushroomexpert.com/pleurotus_pulmonarius.html
- Kuo, M. 2005. "Pleurotus ostreatus: The oyster mushroom." Retrieved from http://www.mushroomexpert.com/pleurotus_ostreatus.html
- Kuo, M. 2006. "Pleurotus populinus: The aspen oyster." Retrieved from http://www.mushroomexpert.com/pleurotus_populinus.html
- Pleurotus dryinus: http://www.messiah.edu/Oakes/fungi_on_wood/gilled%20fungi/species%20pages/Pleurotus%20dryinus.htm
- Pleurocybella porrigens: http://www.messiah.edu/Oakes/fungi_on_wood/gilled%20fungi/species%20pages/Pleurocybella%20porrigens.htm
- Volk, Tom. 1998. "Tom Volk's Fungus of the Month: This month's fungus is Pleurotus ostreatus, the Oyster mushroom." http://botit.botany.wisc.edu/toms_fungi/oct98.html
References Cited
Petersen, R. H. and I. Krisai-Greilhuber. 1996. "An epitype specimen for Pleurotus ostreatus." Mycol. Res. 100:229‒238.
Stamets, P. 2000. Growing Gourmet and Medicinal Mushrooms. 3rd Ed. Berkeley, California: Ten Speed Press.
Vilgalys, R. 1997. "Biodiversity of the oyster mushroom Pleurotus." Mushroom News 1997: 32‒35.