Factors Affecting Soil pH
- Irrigation with high-pH or saline water can cause a gradual increase in soil pH. It is strongly recommended that an irrigation water-quality (pH and salinity) test be done yearly.
- Water drawn from the limestone aquifer is high in bicarbonates and has a liming effect on the soil.•
- Numerous soil amendments, including composts, contain nutrient value and may also have a liming effect resulting in unintended increase in soil pH.
Concern about Soil pH
- Soil pH determines the solubility and bioavailability of nutrients essential for citrus growth and yield.
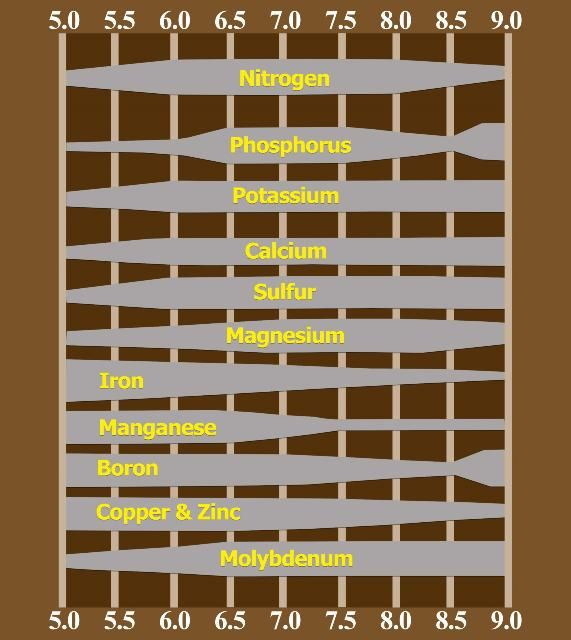
- Research has determined that adjusting soil pH from greater than 7 to the range between 6 to 6.5 increases availability of potassium (K), phosphorus (P), calcium (Ca), manganese (Mn), zinc (Zn) and iron (Fe), improving citrus growth and yield.
- Ferrous iron found in slightly acid soils is the form available to most plant species, but ferric iron in high-pH soils is not. Thus, citrus grown on high-pH soils will be iron-deficient.
- When soil pH reaches 5 or lower, aluminum (Al), Fe, Mn, and/or Zn solubility increase in the soil solution and can become toxic to most citrus.
Management Options
- Management of soil pH and nutrients should include annual soil sampling. Water samples should be taken if elevated soil pH is reported.
- Irrigation water acidification, elemental sulfur application, or use of acidifying fertilizers are recommended to reduce soil pH to the acceptable range.
- Injection of acids into irrigation water high in bicarbonates will reduce soil solution pH, removing soil bicarbonates.
- The current recommendation is to lower irrigation water to a pH between 5 and 6. Soil pH will decrease over time depending on soil buffering capacity and bicarbonate concentration.
- The time required to obtain optimum soil pH (6–6.5) can be 6 to 24 months depending on the soil buffer capacity, moisture, temperature, and aeration.
- In certain cases, elemental sulfur (S) may be applied to the soil. Over time, the soil bacteria will convert the elemental S form into the sulfate form. Sulfuric acid is released in the process, neutralizing hydroxides and bicarbonates.
- Acid-forming fertilizers are positively charged nutrients, such as ammoniacal-N, K, Ca, and Mg, that lower root zone pH after being absorbed by plants.
- Long-term use of ammoniacal fertilizers will also result in lowering the pH by nitrification .
- Sulfate, nitrate, or phosphate ions in fertilizers do not cause soil pH decrease.
pH Test Equipment
Effect of Soil pH on Nutrient Management
- High soil pH (greater than 7.2) causes NH3 volatilization from fertilized soils with ammoniacal-N sources, such as ammonium sulfate, or ammonium-forming fertilizers, such as urea.
- Low soil pH exacerbates nutrient-leaching problems because positively charged nutrients such as ammonium, calcium, magnesium, and potassium adsorbed by soil particles may be replaced by hydrogen protons.
- Nutrient leaching out of the root zone reduces citrus nutrient uptake.
- Caution is recommended to avoid soil pH lower than 5.0.

Credit: Tonya R. Weeks, UF/IFAS
Credit: Nutrition of Florida Citrus Trees. 2nd Edition. T. A. Obreza and K. T. Morgan