Anthracnose fruit rot, caused by fungus in the Colletotrichum acutatum species complex, is an important disease for strawberry worldwide. Other species complexes, such as C. gloeosporioides, are less frequently involved in fruit rot. Although fruit are most frequently affected by C. acutatum, other organs of the plant, including flowers, crowns, leaves, petioles, and roots, are also susceptible.
Pathogens and Symptoms
Anthracnose fruit rot lesions appear as dark, sunken spots on infected fruit (Figure 1). On green fruit, anthracnose lesions are small (1/16–1/8 inch across), hard, sunken, and dark brown or black. Lesions on ripening fruit are larger (1/8–1/2 inch), hard, sunken, and tan to dark brown. During wet weather, the lesions become covered with sticky, light orange ooze composed of millions of spores (conidia) in a mucilaginous matrix (Figure 2). When conditions are favorable for infection, multiple lesions nearly cover the fruit, and lesions may appear on petioles (Figure 3). Strawberry flowers are highly susceptible, turn brown, and remain attached to the plant when infected (Figure 4). Flowers affected by the gray mold fungus Botrytis cinerea may show similar symptoms. Small black spots on green button-sized fruit may also develop from flower infections (Figure 5).

Credit: UF/IFAS GCREC
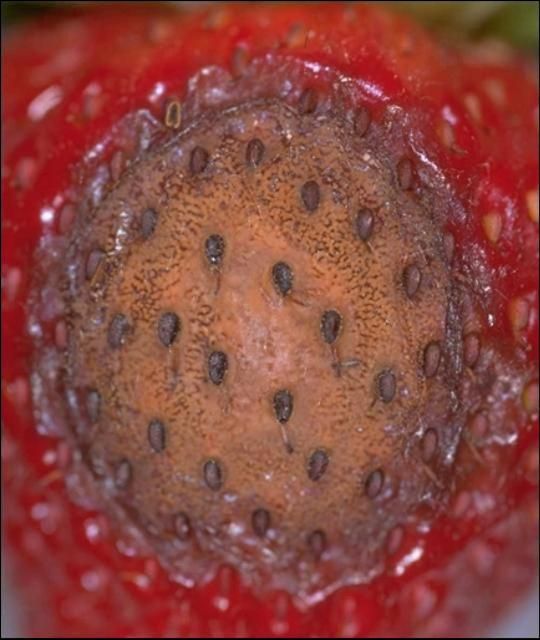
Credit: UF/IFAS GCREC

Credit: UF/IFAS GCREC

Credit: UF/IFAS GCREC

Credit: UF/IFAS GCREC
Disease Development and Spread
The most common way a new strawberry crop is infected in annual production systems such as Florida is through transplants from the nursery. C. acutatum is a strong colonizer of runner plants in the nursery and may be present on the foliage of apparently healthy plants. In some cases, infected runner plants may show visible lesions on the petioles (Figure 3) and roots (see PP128). A new crop may also be infected by inoculum carried over from the previous crop. In the past, traditional post-season crop destruction and cultivation were believed to eliminate inoculum carryover from Florida production fields. However, C. acutatum has been recovered from dead plants left on old plastic during the summer. Thus, if strawberry is planted on old plastic, the inoculum from the old plants could affect the new crop. Moreover, various weeds in and around production fields may also be colonized by C. acutatum from strawberry.
C. acutatum appears to spread first on the foliage, often without causing visible symptoms. A few conidia (asexual spores) are formed on green leaves and petioles, and more are produced as the tissue ages and dies. Conidia are moved from the foliage to the flowers and fruit primarily by splashing water. They then germinate and infect. Developing infections on flowers and fruit produce abundant conidia that are spread to other plants and fields by equipment and harvesters, especially when the plants are wet.
Anthracnose fruit rot development is favored by warm, wet weather. For this reason, epidemics typically occur in the spring when conditions are more favorable. Such epidemics cause serious losses for Florida growers. Crop losses occur mostly in the field since visibly spotted fruit are culled and the development of latent infections postharvest is suppressed by precooling and refrigeration.
Control
Anthracnose fruit rot is best controlled by exclusion (i.e., by not introducing the pathogen into the field). Wherever possible, transplants should be obtained from pathogen-free nurseries. In addition, moving personnel and equipment from diseased to healthy fields without proper cleaning should be avoided. Among the cultivars currently grown in Florida, Sensation® 'Florida 127', 'Florida Brilliance', and ‘Florida Ember’ have higher levels of resistance to anthracnose than 'Florida Medallion', whereas ‘Florida Encore’ is intermediate to these. When highly susceptible cultivars are grown, regular preventive fungicide applications are needed to suppress the disease.
In central Florida, anthracnose management has been based on applications of the broad-spectrum, protectant fungicide captan. Applications can be made at low label rates early in the season because weather conditions are less favorable for disease development at that time. Often a few anthracnose-infected flowers and fruit that appear in late January or early February lead to epidemics during the main harvest in late February and March. During the critical January to March period, protectant fungicides should be applied at higher label rates, and additional fungicides with some curative activity, such as Switch® and Miravis Prime®, may be needed for adequate disease control. Resistance to strobilurin fungicides (Group 11) in Colletotrichum acutatum was first detected in Florida in 2013. Since then, resistant populations have become widespread, and this fungicide group is no longer a reliable option for disease control.
Since anthracnose incidence is highly dependent on weather conditions, fungicide applications can be timed based on key factors, such as leaf wetness duration and temperature during the wetting period. The Strawberry Advisory System (StAS) (http://www.agroclimate.org/ tools/sas) has been developed to advise growers of the need to spray to prevent anthracnose as well as Botrytis fruit rot. StAS provides growers with recommendations for timing as well as suggestions for the selection of the most appropriate product. Disease risk alerts can also be provided to growers via e-mail or text messages.
Research has demonstrated that fungicide sprays can be reduced without affecting disease control or yield when following the StAS recommendations, especially when the more tolerant cultivars, such as Sensation® Florida 127, 'Florida Brilliance' and ‘Florida Ember’ are grown.
A list of fungicide products recommended for anthracnose fruit rot control based on research trials is provided in Table 1.