Introduction
Commercially grown citrus trees are usually composed of two parts: 1) the scion, which is the aboveground portion of the tree that produces the fruit, and 2) the rootstock, which comprises the root system and the lower portion of the trunk. The scion and rootstock are joined via the process of grafting. The most common method of grafting in citrus is budding, in which a single bud from the desired scion variety is inserted into an incision below the bark of the rootstock. After a period of healing, the bud begins to grow and the rootstock stem above the bud union is removed. The result is a citrus plant composed of two genetically different organisms. For more details on the process of grafting, refer to EDIS publication HS1309, Citrus Propagation (https://edis.ifas.ufl.edu/publication/HS1309).
To ensure that citrus nursery stock is horticulturally superior and free of viruses and other graft-transmissible diseases, the Florida Citrus Budwood Registration Program was established under the Florida Department of Agriculture and Consumer Services (FDACS) Division of Plant Industry (DPI) in 1953 and became mandatory for all citrus propagations in 1997. The citrus industry can achieve protection from harmful pathogens and ensure trueness to type only by using certified disease-free plant material for propagation. More details on the citrus budwood program can be found in chapter 7 of this guide, HS1336, Seed and Budwood Production, Transport, and Conservation (https://edis.ifas.ufl.edu/publication/HS1336).
Rootstock Production
Historically, rootstock liners have been produced almost exclusively from apomictic seeds (Figure 1). Other propagation methods, specifically by cuttings and tissue culture (micropropagation), are relevant for several reasons: 1) some rootstock germplasm does not produce sufficient seed; 2) some rootstock germplasm never or infrequently produces nucellar seed; 3) for some rootstock germplasm, no or few seed-source trees are available; and 4) the industry demand for specific superior rootstock germplasm exceeds the available supply. The latter is specifically relevant in the current era of HLB (huanglongbing, a.k.a. citrus greening). In addition, propagation by cuttings or tissue culture reduces the potential risk of disease transmission by seed or by contamination from diseased fruit during the seed extraction process (see below).

Credit: Ute Albrecht, UF/IFAS
Seed Propagation
Nucellar seed. Traditionally, rootstock liners are produced from seeds (Figure 1). In Florida, rootstock seeds are collected from fruit of seed-source trees grown in orchards or in enclosed nursery-owned structures. Alternatively, seeds can be purchased from certified sources. The number of seeds in each fruit varies by rootstock variety and can range from 10–15 for certain mandarin varieties to 40–50 for some trifoliate hybrid varieties. Citrus seeds are usually polyembryonic and produce nucellar embryos that are derived from the maternal tissue (the nucellus) surrounding the embryo sac. This phenomenon is essential for rootstock production as seedlings derived from nucellar embryos will be genetically identical to the mother plant. Sometimes, seedlings will grow from the zygotic embryo of the seed; these plants are called "off-types" and must be rogued because they are genetically different. Off-types are usually easily identified by their different morphology and often inferior growth. In general, many more seeds than rootstock liners must be planted because of germination failure, off-types, and inferiority or death of some seedlings.
Handling of fruit. To avoid potential contamination of seeds with pathogens, particularly Xanthomonas citri subsp. citri, the bacterium causing citrus canker, fruit should always be disinfested in a diluted bleach solution or oxidizing agent composed of hydrogen dioxide and peroxyacetic acid (e.g., OxiDate) prior to extraction. Addition of a nonionic surfactant will ensure surface penetration. Fruit should be completely inundated in the disinfection agent for a minimum of 30 minutes. After disinfection, fruit will need to be washed thoroughly with water. Fruit should be kept in a separate location in the nursery, and any personnel who have been in contact with the fruit prior to disinfection should not enter the nursery or only enter after thorough disinfection.
Seed extraction and storage. Extraction of seeds from the fruit can be done by crushing or by cutting the fruit horizontally just deep enough to enable twisting and moving the two halves apart without injuring the seed. Seeds removed from the fruit will be covered in pulp, which needs to be removed by overnight incubation in an aqueous solution containing the enzyme pectinase, followed by thorough washing in water to remove any adhering pulp.
The pulp-free seeds should be surface-sterilized in a dilute bleach solution or oxidizing agent to ensure they are free of pathogens or other contaminants. If seeds are to be stored for prolonged periods of time, they should be submersed in a 1% 8-hydroxyquinoline sulfate (a fungicide) solution for 10–20 minutes, air dried, and packaged for storage at 39ºF (4ºC). When properly treated and packaged in sealed plastic bags, seeds can be stored in a refrigerator for several months with little loss in viability. Seeds must be completely dry before storage to eliminate any diseases. Drying of seeds followed by cold storage is recommended, even if seeds are not to be stored long-term, because it will ensure germination upon planting. Germination will be delayed if seeds are planted without drying and at least a short period of cold storage. Pre-plant soaking of dried seeds in aerated water maintained at 85°F (29ºC) for 24 hours will increase total germination and uniformity and will shorten germination time.
Seed coat removal. If seeds need to be germinated immediately, the seed coat can be removed prior to planting. Seed coat removal ensures uniform germination within a few days after planting. Seed coat removal can be done using the following procedure: Incubate 1–1.5 qt of seed in 2 qt of water at 95°F–99°F (35ºC–37ºC) for 1½ hours. With a concentrated sodium hydroxide solution, adjust the pH to 11.5–12.5 and incubate for an additional 1–1½ hours. The pH should remain at 11.5–12.5 and must be adjusted if necessary. After incubation, add 1 qt of NaHCLO (10%) and incubate for an additional 30 minutes. At this point seed coats will start to dissolve. Leave seeds in this solution until seed coats are completely dissolved; this may take up to 1½ hours. Strain seeds, preferably in a colander made of metal, rinse with water, and completely remove seed coats by rubbing against the surface of the colander. Dry seeds completely and store at 4ºC or plant immediately. Use seeds immediately; storage of seeds with seed coat removed should not exceed 1 month.
Seedling Care
Seeds are usually planted into 96 cell flats, trays, or tubes, with one seed in each cell at a depth of 0.25 to 0.5 inch (0.5-1 cm). The potting medium will vary based on the preference of the nursery and may consist of peat in combination with perlite or other additives. Coconut-fiber-based (coir-based) media are also used but require more attention to nutrient management. The potting medium should be sterilized before sowing and should remain moist without overwatering. Periodic applications of seeds and emerging seedlings with a diluted oxidizing agent will prevent any potential diseases, particularly damping-off caused by Phytophthora and citrus canker.
Once seedlings reach an adequate height, they will need to be transplanted into larger containers such as citripots. Only healthy and strong seedlings should be transplanted. Stunted seedlings should be discarded as they will probably never catch up with the larger ones. Special attention should be paid to avoid seedlings with a different leaf morphology or otherwise unusual appearance, because they will likely be off-types (not true to type).
Trays and pots need to be situated on the nursery bench at a minimum height of 3.5 feet (1m) above the ground to avoid contamination from splashing. Seedlings should be arranged by size on the nursery bench in order to receive uniform irrigation, nutrition, and light. This arrangement will ensure a higher percentage of saleable trees and reduction of cost and labor.
Cuttings Propagation
In recent years, nurseries have experienced shortages of seeds for some popular rootstock varieties. Therefore, vegetative propagation methods are required to produce large numbers of genetically identical plants. One method that is popular especially with breeders, who usually do not have seed-source trees available for their new selections, is the propagation of rootstocks through cuttings (Bowman and Albrecht, 2017). For this method, single-node cuttings about 1 inch in length are removed from woody sections of 2-to-5-month-old branches of certified disease-free citrus plants. Using single nodes instead of multiple nodes is more practical because more plants can be produced from the source plant. In general, the leaf should remain attached to the node to allow photosynthates to be delivered to the developing roots but can be reduced to a length of 20%–30% of its original size to avoid overlap with neighboring plants. The basal end of each cutting is then dipped into a rooting powder containing a root-stimulating hormone (usually the synthetic auxin IBA, indole-3-butryic acid) and inserted into premoistened sterilized potting medium (Figure 2). Cuttings need to be kept under high-moisture conditions in order for the plants to survive in the absence of a root system. These conditions can be met by placing plants in an enclosed high humidity environment and/or use of an automated misting system. Under these conditions, a young plant will develop within several weeks after planting. Once cuttings have rooted and plants begun to grow, care can be continued with standard procedures used for seedlings.

Credit: Ute Albrecht, UF/IFAS
Tissue Culture Propagation
Advances in methods for vegetative propagation of citrus rootstocks through tissue culture (micropropagation) have made it possible to economically produce large numbers of genetically identical plants from most rootstock selections. The starting material for establishing micropropagated plants varies depending on the preference of the nursery and can consist of nucellar embryos, stems, or buds, all of which must be derived from disease-free foundation trees. The explants are placed in a nutrient agar medium, which may contain a small amount of growth regulators to accelerate plant regeneration. Once plants are regenerated, they are subcultured every few weeks on fresh nutrient agar medium to generate multiple shoot clusters (Figure 3).

Credit: Beth Lamb, Phillip Rucks Citrus Nursery
After an elongation phase, clusters are separated into individual shoots (Figure 3). Depending on the preference of the nursery, individual plants are rooted on agar medium before transplanting, or they are directly rooted into potting medium (Figure 4). After an acclimatization period under high humidity conditions, roots will develop and the young rootstock plant can be managed using the same procedures applied for seedlings. Use of tissue culture allows the rapid propagation of large numbers of identical and disease-free plants and has become routine for many fruit crop systems.

Credit: Ute Albrecht, UF/IFAS
Concerns of Cuttings and Tissue-Culture Propagation
Despite many advantages of tissue-culture propagation, citrus nurseries in Florida prefer rootstock liners that originate from seed over those that originate from tissue culture. In addition to the anticipated higher costs, there is concern these trees will have an inferior root system and inconsistent vigor.
The root systems of plants generated from seeds or from cuttings and tissue culture are quite different. Contrary to plants grown from seed which generally have a single and well-defined taproot from which multiple smaller roots arise, plants generated from cuttings and by tissue culture have multiple adventitious or lateral roots instead of a taproot (Figure 5).
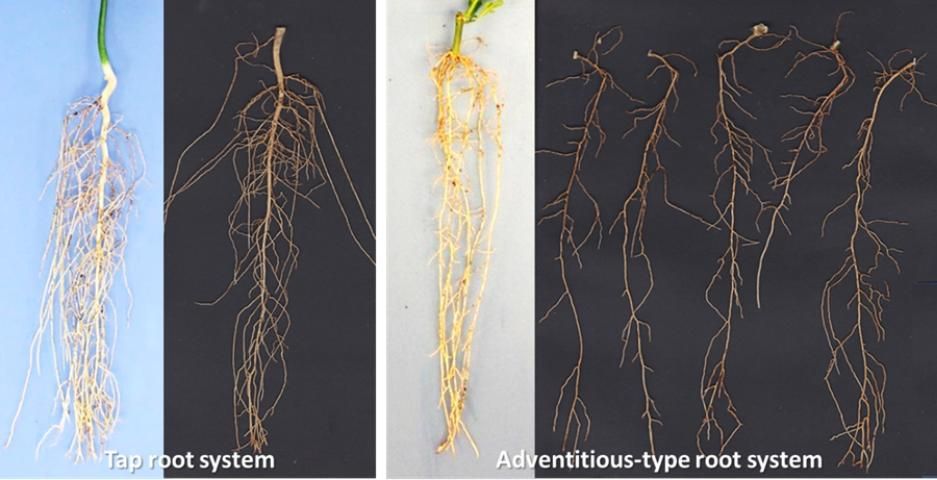
Credit: Ute Albrecht, UF/IFAS
Detailed studies on different rootstock germplasm demonstrated that the number of adventitious roots can range from 2 to more than 10 roots per plants (Albrecht et al., 2017). The high number of adventitious roots compared with a single taproot usually results in a higher number of smaller lower-order roots, and consequently increased total and specific root length (root length divided by root dry weight) of these plants. Due to the different propagation process, plants from cuttings and tissue culture also have a significantly smaller root-to-shoot ratio compared with seedlings during the early stages of development. The larger specific root length and lower root-to-shoot ratio are indications of a higher water and nutrient uptake efficiency of these plants compared with seedlings. Consequently, nurseries will have to adjust their management practices based on the method by which rootstock liners are produced.
Despite these significant differences during the early growth stages, as liners are grafted and continue to grow, differences in total root length, fibrous root numbers, and root-to-shoot ratio become less evident between plants on seedling, cutting, and tissue-culture-generated rootstocks (Albrecht et al., 2020). In fact, at this stage, differences are observed primarily based on the genetic propensity of the rootstock variety rather than based on the method by which they were propagated. The same was found after two years of field growth (Pokhrel et al., 2021). Whether the method of rootstock propagation will influence tree growth in the longer-term under commercial field conditions is still under investigation.
References
Albrecht U., Bodaghi S., Meyering B., Bowman K.D. (2020). Influence of rootstock propagation method on traits of grafted sweet orange trees. HortScience 55:729-737. https://doi.org/10.21273/HORTSCI14928-20
Albrecht U., Bordas M., Lamb B., Meyering B., and Bowman K.D. (2017). Influence of propagation method on root architecture and other traits of young citrus rootstock plants. HortScience 52:1569-1576. https://doi.org/10.21273/HORTSCI12320-17
Bowman K.D. and Albrecht U. (2017). Efficient propagation of citrus rootstocks by stem cuttings. Sci. Hort. 225:681–688. https://doi.org/10.1016/j.scienta.2017.07.049
Pokhrel, S., B. Meyering, K.D. Bowman, U. Albrecht. 2021. Horticultural attributes and root architectures of field-grown ‘Valencia’ trees grafted on different rootstocks propagated by seed, cuttings, and tissue culture. HortScience 56:163-172. https://doi.org/10.21273/HORTSCI15507-20