Colletotrichum acutatum is widely known as a fruit rot pathogen, but it also infects other strawberry organs, including the roots.
Pathogens and Symptoms
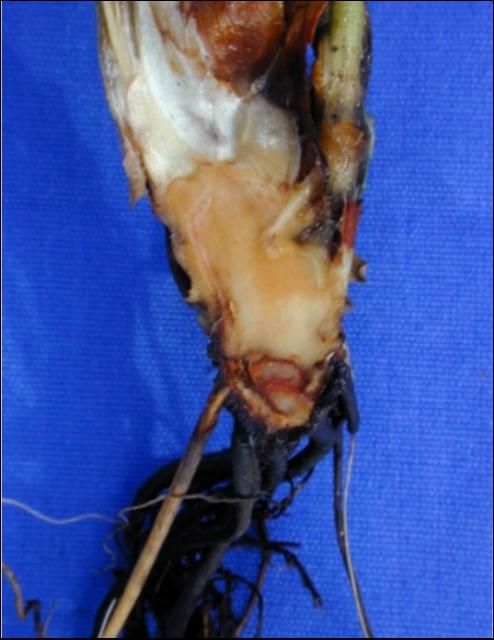
Transplants with infected root systems often grow poorly or fail to become established after overhead irrigation is withdrawn. Few functional roots are found on infected plants even 1 to 2 weeks after transplant (Figure 1). Old structural roots are brown or black with few feeder roots, whereas new roots develop brown lesions, die back from the tip, or fail to emerge from the crown (Figure 2). In severe cases, C. acutatum enters the crown, causing a basal crown rot and eventually killing the plant (Figure 3). Surviving plants are stunted or variable in size, flower late, and produce a poor early crop (Figure 4). Infected plants may recover during the cool winter months and produce normally in February and March, if an outbreak of anthracnose fruit rot does not occur.

Credit: UF/IFAS GCREC

Credit: UF/IFAS GCREC
Credit: UF/IFAS GCREC

Credit: UF/IFAS GCREC
Disease Development and Spread
C. acutatum frequently colonizes leaves and petioles of runner plants in the nursery. Symptoms may not be visible in the nursery environment, but if inoculum is allowed to build up and the weather is favorable, lesions may develop on the flowers and fruit of day neutral cultivars and on the petioles of susceptible short-day cultivars (Figure 5). The roots of the plants are presumably contaminated by this inoculum during normal digging, trimming, and packing operations in the nursery.

Credit: UF/IFAS GCREC
Early in the season, disease spread below ground is unlikely since the root systems are relatively isolated; however, above-ground spread in the foliage does occur and may be facilitated by overhead irrigation during establishment. Observations suggest that disease severity is influenced by the degree of plant stress during establishment. Even cultivars that are only moderately susceptible to anthracnose fruit rot, e.g., 'Sensation® Florida 127', and 'Florida Brilliance', are susceptible to root necrosis.
Control
Diseases caused by C. acutatum are best controlled by exclusion (not introducing the pathogen into the field). Therefore, transplants should be purchased from a reputable source. However, this does not guarantee disease-free material. For that reason, transplants should be inspected for petiole lesions caused by C. acutatum (Figure 5).
When the pathogen is known to be present and susceptible cultivars are being grown, pre-plant fungicide dips may be used to suppress disease development. Abound®, Oxidate®, Actinovate®, Switch®, and Zivion are currently labeled for this use (Table 1). These products have been tested at UF/IFAS GCREC—Balm by dipping naturally infected runner plants for 5 minutes just before planting. Plants dipped in Switch survived better and produced higher marketable yields in all trials. However, there are reports of phytotoxicity when plants were dipped longer than 5 min or when plants were not planted right after dipping. Actinovate has worked well in some trials but not others and Oxidate was not effective in our trials. Abound has performed well in our trials until the 2013–2014 season, when control failure was due to the emergence of C. acutatum populations resistant to the strobilurin fungicides. Thus, dipping in Abound is no longer recommended. Zivion S has recently been registered for dip treatment of strawberry plants and has performed well in most of our trials. Growers should consider dip treatments mainly for cultivars that are known to be highly susceptible and/or when transplants are from nurseries with a history of C. acutatum.
Measures that reduce plant stress during establishment may also reduce the severity of root necrosis disease. Whenever possible, strawberries should be planted in the morning to avoid high temperatures and drying conditions that occur in the afternoon. After setting, overhead irrigation should be started as soon as possible to prevent transplants from wilting on the hot plastic. After 7 to 8 days, plant response should be used to determine if overhead irrigation is still necessary. Irrigation should be continued in the afternoon if drying conditions are encountered or the plants wilt when water is withdrawn. If hot weather is expected after overhead irrigation is withdrawn, consider spraying the beds with Surround®, which reduces heat stress by coating the plants and plastic with white kaolin clay.
Literature Cited
Forcelini, B. B., Seijo, T. E., Amiri, A., and Peres, N. A. 2016. "Resistance in strawberry isolates of Colletotrichum acutatum from Florida to quinone-outside inhibitor fungicides". Plant Dis. 100:2050–2056.