Mastitis can become a costly health problem for small ruminant producers. Thus, proactive testing may help reduce treatment and management costs.
There are two types of mastitis: clinical and subclinical. Clinical can be visually recognized while subclinical is more common but not as easy to identify. To determine if a small ruminant has mastitis, a California Mastitis Test (CMT) can be conducted.
The CMT is a simple and relatively inexpensive test, allowing small ruminant producers to quickly know if their animals have mastitis. When an infection occurs, white blood cell numbers increase in the udder to help fight and stop the spread of infection. The CMT reagent reacts with milk containing high levels of white blood cells (i.e., somatic cells), forming a gel. The greater the number of white blood cells present, the thicker and more gel-like the resulting solution will be. Keep in mind that the milk of early- and late-lactation small ruminants can react to the CMT and form a gel even in the absence of subclinical mastitis. Thus, results during these specific phases of the lactation cycle may not always be reliable. In case of a positive CMT, it is recommended that additional laboratory tests be conducted to determine which organisms are causing the infection. Below are the steps to follow to use CMT on small ruminants.
CMT Supplies
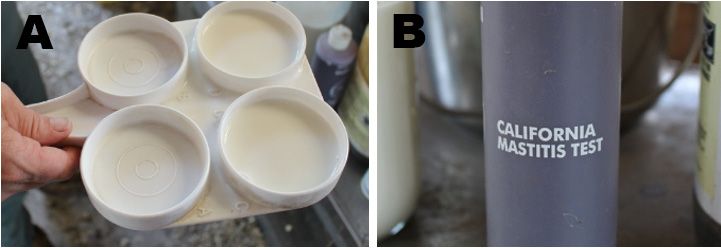
Credit: Credit:
A Step-by-Step Guide
STEP 1
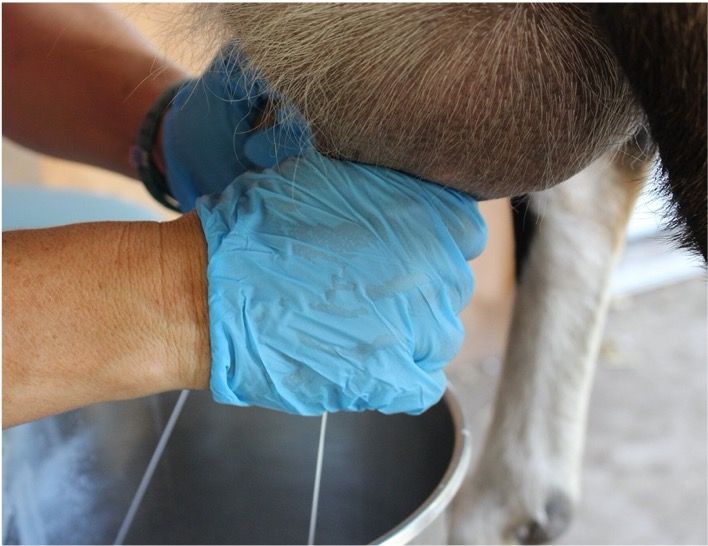
Milk samples from each quarter are collected in a clean CMT paddle. Be sure to discard the first stream of milk, then fill each cup with milk (1 teaspoon–2 cc).
You can tilt the paddle to discard excess milk until equal volumes remain in each well.

Credit: Santos et al. 2015
STEP 2
Slowly add an equal amount of CMT solution(1 teaspoon–2 cc) to each well in the paddle. Tilt paddle back until milk is halfway between the inner and outer circles.

Credit: Santos et al. 2015
STEP 3
Gently swirl the CMT paddle in a circular motion to homogenize the solution.

Credit: Santos et al. 2015
STEP 4
Results can be observed within 10 seconds and should be read right away, as the reaction may change.

Credit: Santos et al. 2015
STEP 5
Remember to always rinse the CMT paddle after each test.
Reading and Interpreting Results
Results can be interpreted differently based on the individual tester. Producers should familiarize themselves with what a negative or positive CMT result looks like. If somatic cells are present, the solution will thicken to form a gel of differing degrees of thickness. Record the results and contact a veterinarian if CMT results are positive. Refer to the table below to interpret the results.

Credit:

Credit:
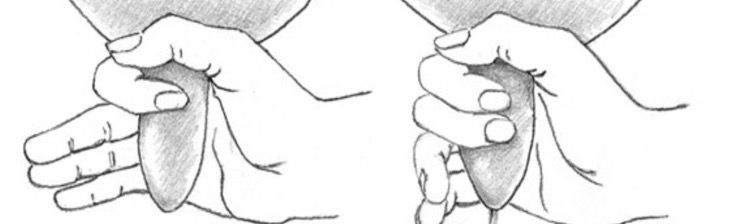
How to Properly Hand-Milk Goats
Producing High-Quality Milk
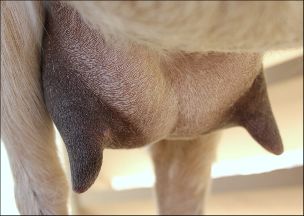
The keys to producing quality milk are a sanitary environment, properly cleaned and maintained milking supplies/equipment, proper milking procedure, and does with healthy udders.
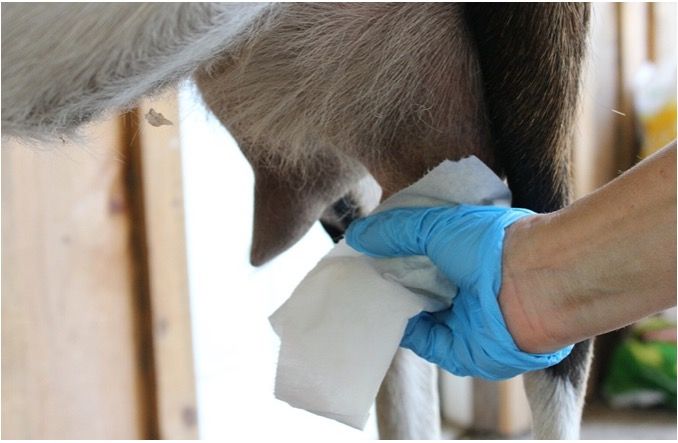
Milking clean, dry, and properly stimulated teats allows the most efficient harvesting of milk and reduces the risk of udder infections (mastitis).

Credit: undefined
Milking Environment
Housing conditions should be one of the first considerations in creating a clean environment for harvesting milk. When dairy goats lie down, their udders are in direct contact with the floor. It is crucial that this surface is clean, dry, and comfortable. The risk of mastitis increases greatly in dirty environments. Adequate bedding materials such as straw, sawdust, or sand bedding should be cleaned of manure daily and supplemented as needed. Resting areas should be in spaces that are not prone to flooding or moisture accumulation. A clean, safe, and stress-free environment is very important for efficient milking.
When the teats of a dairy animal are properly stimulated, the hormone oxytocin is released to cause the milk letdown. Fear or stress prior to milking can interfere with oxytocin release and disrupt milk letdown. For this reason, animals should not be stressed entering the milking area, which should be a calm and quiet place. Good milkers are patient with animals and pay attention to details. Aggressive handling, long waiting periods, or any changes in milking routine can interfere with milk letdown. Cooling systems that include soakers and fans should be used during extreme summer conditions. Keeping the animals comfortable will increase the overall milk yield and quality.
Milking Supplies
- Milk stand strip cup
- Stainless steel bucket
- Cloth/Paper towels
- Pre/Post-dipping solution
- Disposable filters and strainer
- Glass jars for milk storage
- Gloves