Purpose
The purpose of this publication is to inform readers about horseshoe crabs, their biology, distribution, natural history, culture methods, diseases and parasites, purposes for aquaculture, and markets. The target audience of this publication is all interested in horseshoe crabs, with an emphasis on those wanting information about their culture methods and potential markets.
General Description
The American horseshoe crab, Limulus polyphemus (Figure 1), is a member of the phylum Arthropoda, subphylum Chelicerata, and class Merostomata (Pearse et al. 1987; Sekiguchi and Shuster, 2009). Despite their name, horseshoe crabs are not true crabs but relatives of arachnids (e.g., spiders and scorpions). Horseshoe crabs are protected by a hard, brown exoskeleton called a carapace, that is only molted during the larval and juvenile life stages. The underside of the abdomen houses the book gills, mouth, and ten legs. The long tail, also known as a telson, allows the animal to flip over when stuck on its back. In addition to the pair of compound eyes embedded into the hard carapace, multiple microscopic eyes called “photoreceptors” are present along the length of the shell and tail.

Credit: Alice Mary Herden
Geographic Distribution and Habitat
Although there are four horseshoe crab species worldwide, the American horseshoe crab Limulus polyphemus is primarily found in the Atlantic Ocean along the east coast of North America. The species ranges from Maine down the coast to Mexico, but is absent between Texas in the United States and Tabasco in Mexico. They mainly inhabit estuarine areas; however, juveniles and adults can venture into oceanic environments (Smith et al. 2017). Embryo development occurs in the intertidal zone of sandy beaches. Sandy or muddy bottom habitats are necessary for burrowing and benthic feeding.
Natural History
As an estuarine species, horseshoe crabs experience a considerable variation in water quality parameters. Adults tolerate temperatures ranging from 23°F to 95°F and salinities ranging from 5 ppt (g/L) to 35 ppt (g/L). The species is sexually dimorphic, with males smaller than females. Sexual maturity begins around nine years of age, and the lifespan is 15 to 20 years (Pearse et al. 1987).
During the spring and fall, horseshoe crabs migrate inshore to spawn during the highest of high tides of new and full moons. The exact time this occurs varies by latitude (Figure 2; Brockmann and Johnson 2011). A male will attach to the female using modified claws that resemble boxing gloves. The male will then deposit sperm over the eggs as the female lays them in the sand within a hole she digs during nesting (Figure 3). Some males fertilize eggs without attaching to the female. Each female lays up to 88,000 eggs within a nesting season. Eggs are laid in clumps and are usually a green color (Figure 4; Smith et al. 2017). The free-swimming trilobite larvae hatch from the eggs after two to four weeks.

Credit: Florida Sea Grant, Brittany Scharf

Credit: Alice Mary Herden, UF/IFAS Extension Hernando County volunteer

Credit: U.S. Fish and Wildlife Service, Gregory Breese
Horseshoe crabs spend time burrowed under the substrate to protect themselves from receding tides, predation, extreme temperature fluctuations, and to feed. They are benthic predators, which means that they find their prey on the bottom of the ocean. Adults primarily consume mollusks, worms, and crustaceans. Juveniles feed on benthic and suspended organic particles, whereas trilobite larvae get their first nutrition from the yolk (Botton 2009).
Culture Techniques
The discussion of horseshoe crabs in aquaculture is a recent development. Some studies have evaluated culture methods for each life stage. This publication will review these known culture parameters in non-aquaculture settings and the natural history of this organism. The intended project outcome will dictate the life stage and culture technique selected.
Broodstock
Eggs are acquired through artificial breeding, electrical stimulation, or collection of wild eggs. Naturally spawned eggs have a higher fertilization and are easy to collect if proper permits are obtained (Xiu et al. 2021).
Hatchery
Once fertilized, the eggs should be kept in McDonald-style hatching jars. Finger bowls can be used; however, eggs experience less agitation in finger bowls and are more prone to fungal disease than those placed in McDonald jars. Each McDonald jar can hold 300 to 500 eggs, and a flow rate of 40L/min should be maintained for each jar (Schreibman and Zarnoch 2009). Water should be free of heavy metals or have very low concentrations. Heavy metals and other pollutants have had adverse effects on horseshoe crab eggs. The ideal water quality limits for egg development are a temperature range of 77°F to 91°F and a salinity range of 20 ppt (g/L) to 40 ppt (g/L). Although larvae experience varying conditions in their natural environment, higher temperatures can be detrimental to growth. Furthermore, the pH in larval culture should be kept between 7.5 and 8.8 (Botton and Itow 2009). These factors account for the considerable variation during development in the intertidal zone.
After one month of incubation, the eggs hatch, and trilobite larvae emerge. They should be transported immediately to a downweller system (water flows from the top downwards and holds eggs on a screen), where they will remain for three months. Water quality (Table 1) should be close to the horseshoe crabs’ natural environment. Temperature should be 68°F to 77°F, salinity 27 ppt (g/L) to 33 ppt (g/L), pH 7.5 to 8.8, ammonia (NH3-N) less than 1.0 ppm (mg/L), nitrite less than 0.6 ppm (mg/L), and dissolved oxygen (DO) concentration greater than 5 mg/L. Water quality parameters may vary slightly, but again, if possible they should closely reflect conditions in the crabs’ natural environment. Water quality should be measured daily. During this stage of culture, trilobite larvae look like the adult horseshoe crab, although they lack the long telson (FFWC 2020a; GARFO 2018). Trilobite larvae get their nutrition from their yolk and do not need food at this stage. After their first molt in culture, they should be fed brine shrimp nauplii, around 450 µm in size.
Table 1. A summary of acceptable water quality parameters from Tinker-Kulberg et al. 2020.
After three months, the larvae should be moved to a recirculating aquaculture system (RAS). Tanks can vary in size, but it is recommended that tank bottoms be covered in sand. The sandy bottom provides a substrate for the horseshoe crabs to burrow into and minimizes epibiont growth (an epibiont is an organism that lives on the surface of another organism). Juveniles are fed brine shrimp or clams three times per week (1.5% of horseshoe crab body weight) during this life stage. Juveniles are then moved to a second RAS with a sand-substrate bottom, where they will remain for another three months. In this system, the horseshoe crabs are fed chopped clams and commercial fish pellets to increase their protein intake. Once horseshoe crabs reach four to 12 months of age, juveniles can be released back into the wild for stock enhancement. Juveniles are typically not held longer than six months of age because they can experience high mortality rate (Schreibman and Zarnoch 2009).
Nursery
Horseshoe crabs are juveniles until they reach sexual maturity and must molt their carapace to grow. The rate of molting decreases as crabs grow and ceases upon maturity. Light influences molting, and therefore, a 12-hour light-dark cycle should be mimicked for increased growth and healthy molts. Sand-bottom tanks also minimize fungus and epiphyte growth on the horseshoe crab's shell. During this juvenile stage, horseshoe crabs can be fed macroalgae and phytoplankton, consistent with their diet in the natural environment. A diet containing 35%–40% protein is essential, so chopped clam meat and sinking commercial fishmeal pellets (35%–40% protein content) should be considered (Schreibman and Zarnoch 2009).
Growout
Horseshoe crabs are not cultured for size, so “growout” depends on the goal of the consumer. The bait industry, aquarium trade, and research purposes can also dictate the size of the desired horseshoe crab. Because of high mortality rates, juvenile horseshoe crabs for stock enhancement purposes are typically released back into the wild from 4 to 12 months of age, when they are at least 6 mm in size.
Adult horseshoe crabs cultured for biomedical purposes can be maintained in outdoor pens or recirculating aquaculture systems. There are pros and cons when considering the method of adult culture. Selecting outdoor net pens has reduced infrastructure needs, feed costs, and operational costs. However, fluctuations in temperature, natural food availability, and oxygen contribute to mortality within the system. Additionally, collecting the horseshoe crabs within the pens can be difficult due to their burrowing behaviors. Indoor RAS systems regulate temperature and other water quality parameters and allow for disease and nutrition monitoring. However, this method does come at a higher operational cost (Tinker-Kulberg et al. 2020b).
Outdoor Pens for Adult Culture
Outdoor net pens can be made of PVC pipe and crab trap netting within a semi-tidal saltwater pond. These cages must be secured to the bottom of the pond so that the crabs can forage naturally without escaping, especially since horseshoe crabs tend to burrow down into the sediments. The net pens should extend out from the shore with a minimum depth of four feet. Additionally, net pens should be spaced at least four feet apart so water can exchange freely and thoroughly flush the pen. Within these net pens, stock horseshoe crabs at a density of one per 6 ft2. Too high of a density can negatively impact the health of the horseshoe crabs. The horseshoe crabs should also be rotated weekly among the net pens to prevent sediment pollution. In addition to the natural forage, horseshoe crabs can be fed glass minnow and krill one time per week (Tinker-Kulberg et al. 2020b).
Recirculating Aquaculture Systems for Adults Used for Hemolymph Harvest
Adult horseshoe crabs can also be cultured in RAS (Figure 5; Tinker-Kulberg et al. 2020b). Rectangular tanks measuring 4ft x 6ft x 1 ft are ideal holding tanks and are stocked at one per 3 ft2. A separate holding tank connected to the system works as an isolation tank for new crabs or crabs that recently underwent catheter surgery. The RAS should include an UV sterilizer, a biofilter, clarifying tanks, and adequate aeration. A 12-hour photoperiod and proper water quality must be maintained (Tinker-Kulberg et al. 2020b).
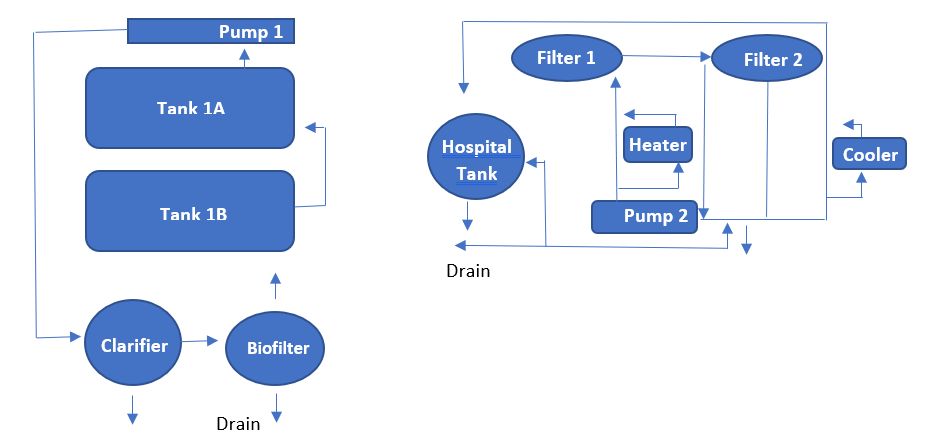
Approximately 600,000 horseshoe crabs are wild-caught and bled for hemolymph annually. If hemolymph is the aquaculture goal for biomedical purposes, surgically implant a catheter into the back of the horseshoe crab. To meet the current biomedical demand, approximately 60,000 horseshoe crabs would need to be bled 12 to 24 times per year within an aquaculture setting. However, it is not recommended to bleed more than 4 times per year. A feed comprised of beef gelatin, collagen, poultry biproduct, and natural supplements is ideal for quality Limulus amoebocyte lysate (LAL) derived from horseshoe crabs in RAS systems (Tinker-Kulberg et al. 2020c).
Disease
The horseshoe crab is a hardy species, tolerant to wide changes in water quality and many diseases. However, many diseases and parasites can occur. Captive horseshoe crabs can experience panhypoproteinemia usually three to four weeks after capturing from the wild. The total protein levels drop below 3.4–11.7 g/dL, possibly due to poor nutrition. This condition includes anorexia and lethargy; the hemolymph becomes clear instead of a healthy blue. Other non-infectious challenges result from poor water quality, molting problems, and physical injuries (puncture wounds, fractures in the carapace, crushing of exoskeleton). When choosing crabs for broodstock, choose only those with brown shells free of epibionts. Quarantine horseshoe crabs for a significant amount of time to monitor them during those first three to four weeks visually and through hemolymph testing.
Diseases of the shell are the most common infectious diseases. A greenish to grayish discoloration could result from a pathogenic green alga. Fungal diseases are present in captive crabs. Cyanobacteria, genus Oscillatoria, can be found on the gill surface or in gill tissue. Other bacteria species isolated from the horseshoe crab include Beggiatoa sp., Leucothrix sp., Vibrio sp., Flavobacterium sp., Pseudomonas sp., and Pasteurella sp. It is important to note that where LAL reduces the chance horseshoe crabs develop sepsis, it does not entirely protect the crab from septicemia (Nolan and Smith 2009).
Parasites can colonize horseshoe crabs: protozoans, trematodes, nematodes, and turbellarian flatworms. Two main parasites of the horseshoe crab are Microphallus limuli, a digenic trematode, and Bdelloura candida, a turbellarid flatworm (Nolan and Smith 2009). Microphallus limuli is a parasite of the herring gull (Larus argentatus); the horseshoe crab is the second intermediary host. It can be isolated from the connective tissue, brain, muscle, or eye of both juvenile and adult horseshoe crabs. Bdelloura candida can be found in the gill leaflets. It receives its nutrition from the horseshoe crab’s hemolymph through lesions on the gills (Nolan and Smith 2009).
Clinically examining horseshoe crabs is complex, challenging disease detection. Veterinarians must review histopathological sample tissues, draw hemolymph for clinical chemistry and culture, use fecal floats, visualize wet mounts of carapace or gill shavings, and use radiology techniques to assess horseshoe crab health (Nolan and Smith 2009).
Preventive measures (i.e., best water quality and nutrition, and routine health checks) are the best way to ensure the health of the horseshoe crab culture. There is no definitive treatment for microbial diseases, and treatment success has yet to be reported. The FDA has approved the antibiotic oxytetracycline for invertebrates. There are three methods to combat external parasites: freshwater, formalin (combined with freshwater), or acetic acid baths. The only other method to prevent the spread of disease is to euthanize afflicted horseshoe crabs; necropsies can be performed on crabs when no other technique can determine the cause of illness (Nolan and Smith 2009).
Market
Horseshoe crabs are very important as bait for eel and whelk fisheries, and they are also marketed for the aquaria, research, and biomedical and pharmaceutical industries (FFWC 2020b; Novitsky 2009; Tinker-Kulberg et al. 2020a; Walls et al. 2002). It is estimated that horseshoe crabs contribute $123 million to regional economies and $175 million nationally (Walls et al. 2002). In 2007, live horseshoe crabs sold for $2.00–$2.50 per crab (Kreamer and Michels 2009). Furthermore, LAL from horseshoe crab blood is used in the biomedical field to test intravenous fluids, vaccines, and other medical equipment for pathogens (Tinker-Kulberg et al. 2020a).
Farming horseshoe crabs has economic potential. Their blood has sold for $15,000 a quart (0.95 L), and yearly profits are estimated at $50 million per year (PBS 2008). Typically, horseshoe crabs are harvested from the wild, and 30% of the hemolymph is drawn three to four times a year. Keeping horseshoe crabs in an RAS allows the ability to consistently draw lower volumes of blood onsite while monitoring the horseshoe crabs in “hospital tanks” to ensure survivability. This is a more economical solution that supports the conservation efforts of wild populations and individual horseshoe crab health.
There is a synthetic LAL alternative that has been investigated to replace horseshoe crab hemolymph but is not used worldwide and is pending approval in many countries (Maloney 2018). If this were to be approved and become commercially available, this could certainly alter the demand for horseshoe crab hemolymph.
Conclusion
Although horseshoe crab aquaculture can be challenging, the medical, economic, and conservation influences are beneficial. Adult horseshoe crabs have been successfully cultured in RAS at a pilot scale. However, there is still further information needed for culturing all life stages including optimizing feeding, best water quality parameters, and defining captive spawning techniques (Tinker-Kulberg et al. 2020a). Horseshoe crab aquaculture is a newly developed field. Most studies were published within the last ten years, and the RAS system design was published in 2020. Most of the information on aquaculture of horseshoe crabs has been attained from wild-collected individuals. Further research on captive breeding to contribute to conservation needs and the demand for hemolymph is justified.
References
Brockmann, H. J., and S. L. Johnson. 2011. “A Long-Term Study of Spawning Activity in Florida Gulf Coast Population of Horseshoe Crabs (Limulus polyphemus).” Estuaries and Coasts 34:1049–1067. https://doi.org/10.1007/s12237-011-9419-1
Maloney, T., R. Phelan, and N. Simmons. 2018. “Saving the Horseshoe Crab: A Synthetic Alternative to Horseshoe Crab Blood for Endotoxin Detection.” PLoS Biology. 16 (10): e2006607. https://doi.org/10.1371/journal.pbio.2006607
Nolan, M. W., and S.A. Smith. 2009. “Clinical Evaluation, Common Diseases, and Veterinary Care of the Horseshoe Crab, Limulus polyphemus.” InBiology and Conservation of Horseshoe Crabs., edited by J. Tanacredi, M. Botton, and D. Smith. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_30
Novitsky, T. J. 2009. “Biomedical Applications of Limulus Amoebocyte Lysate.” In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith. Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_20
PBS. 2008. “The Benefits of Blue Blood.” PBS. https://www.pbs.org/wnet/nature/crash-a-tale-of-two-species-the-benefits-of-blue-blood/595/
Schreibman, M. P., and C. B. Zarnoch. 2009. “Aquaculture Methods and Early Growth of Juvenile Horseshoe Crabs (Limulus polyphemus).” In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith, Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_31
Sekiguchi, K., and C. N. Shuster Jr. 2009. “Limits on the Global Distribution of Horseshoe Crabs (Limulacea): Lessons Learned from Two Lifetimes of Observations: Asia and America.” In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith, Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_1
Shuster, C. N., Jr., and K. Sekiguchi. 2009. “Basic Habitat Requirements of the Extant Species of Horseshoe Crabs (Limulacea).” In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith, Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_7
Smith, D. R., H. J. Brockmann, M. A. Beekey, T. L. King, M. J. Millard, and J. Zaldívar-Rae. 2017. “Conservation Status of the American Horseshoe Crab, (Limulus polyphemus): A Regional Assessment.” Reviews Fish Biology Fisheries 27: 135–175. https://doi.org/10.1007/s11160-016-9461-y
Tinker-Kulberg, R., K. Dellinger, T. E. Brady, L. Robertson, J. H. Levy, S. K. Abood, F. M. LaDuca, C. L. Kepley, and A. L. Dellinger. 2020a. “Horseshoe Crab Aquaculture as a Sustainable Endotoxin Testing Source.” Frontiers in Marine Science 7:153. https://doi.org/10.3389/fmars.2020.00153
Tinker-Kulberg, R., A. L. Dellinger, L. C. Gentit, B. A. Fluech, C. A. Wilder, I. L. Spratling, D. J. Stasek, et al. 2020b. “Evaluation of Indoor and Outdoor Aquaculture Systems as Alternatives to Harvesting Hemolymph from Random Wild Capture of Horseshoe Crabs.” Frontiers in Marine Science 7:568628. https://doi.org/10.3389/fmars.2020.568628
Tinker-Kulberg, A. Dellinger, T. E. Brady, L. Robertson, M. K. M. Goddard, J. Bowzer, S. K. Abood, C. Kepley, and K. Dellinger 2020c. “Effects of Diet on the Biochemical Properties of Limulus Amebocyte Lysate from Horseshoe Crabs in an Aquaculture Setting.” Frontiers in Marine Science 7:541604. https://doi.org/10.3389/fmars.2020.541604
Walls, E. A., J. Berkson, and S. A. Smith. 2002. “The Horseshoe Crab, Limulus polyphemus: 200 Million Years of Existence, 100 Years of Study.” Reviews in Fisheries Science, 10 (1): 39–73. https://doi.org/10.1080/20026491051677
Xiu, P., H. Bai, X. Xie, C-C. Wang, X. Huang, X. Wang, M. Zhang, et al. 2021. “Tri-Spine Horseshoe Crab Aquaculture, Ranching and Stock Enhancement: Perspectives and Challenges.” Frontiers in Marine Science 8:608155. https://doi.org/10.3389/fmars.2021.608155
Additional Readings
Botton, M. L. 2009. The Ecological Importance of Horseshoe Crabs in Estuarine and Coastal Communities: A Review and Speculative Summary. In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith, Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_3
Botton, M. L., and T. Itow. 2009. “The Effects of Water Quality on Horseshoe Crab Embryos and Larvae.” In Biology and Conservation of Horseshoe Crabs, edited by J. T. Tanacredi, M. Botton, and D. Smith, Springer, Boston, MA. https://doi.org/10.1007/978-0-387-89959-6_27
Carmichael, R. H., and E. Brush. 2012. “Three Decades of Horseshoe Crab Rearing: A Review of Conditions for Captive Growth and Survival.” Reviews in Aquaculture 4:32–43. https://doi.org/10.1111/j.1753-5131.2012.01059.x
Florida Fish and Wildlife Conservation Commission. 2020. Rule: 68B-46.002 Horseshoe Crabs Harvest Restrictions: License Requirements, Gear Specifications, Daily Bag and Possession Limits. Florida Administrative Code & Florida Administrative Register. https://www.flrules.org/gateway/ruleNo.asp?id=68B-46.002
Florida Fish and Wildlife Conservation. Facts About Horseshoe Crabs and FAQ. Florida Fish and Wildlife Conservation. Accessed October 2020 from https://myfwc.com/research/saltwater/crustaceans/horseshoe-crabs/
Greater Atlantic Regional Fisheries Office. 2018. “Horseshoe Crabs: Managing a Resource for Birds, Bait, and Blood.” NOAA Fisheries. https://www.fisheries.noaa.gov/feature-story/horseshoe-crabs-managing-resource-birds-bait-and-blood