This is the third publication in the cellular agriculture series that provides information to seafood industry producers on production strategies and considerations for developing novel cell-based seafood products.
Introduction
With the increasing need for alternative and sustainable protein sources to feed the growing population, the number of companies investing in the development of cell-based seafood and other food products is rising. Ensuring food safety stands as a primary concern when integrating new technology into food production processes; therefore, cell-based products must receive regulatory approvals prior to release onto the market. In the first and second publications of this EDIS series, we discussed background information on cell-based seafood manufacturing techniques, the challenges of the industry, and the manufacturing advantages of lean white fish for cell-based seafood production. In this publication, we provide information on the current regulatory framework for cell-based seafood production in the United States, food safety considerations, and the path forward for cell-based seafood products to get approved for sale. Additionally, drawing on the available science-based risk analysis methods for seafood production such as Hazard Analysis Critical Control Point (HACCP), a hazard analysis to identify the potential concerns that can potentially compromise the safety of cell-based seafood products, is provided.
Which federal agency has jurisdiction over cell-based seafood production in the United States?
In 2019, the U.S. Food and Drug Administration (FDA) and the United States Department of Agriculture’s Food Safety and Inspection Service (USDA-FSIS) established a joint regulatory framework for the oversight of cell-based food products derived from livestock, poultry, fish, shellfish, and Siluriform fish. Under this agreement, the FDA has the sole authority to oversee all production stages for cell-based seafood except for the Siluriform species and game meat (FDA 2019). Based on this agreement, cell-based seafood producers should complete a pre-market consultation with the FDA, which will address the processes and resulting products, including the biological materials used. If these consultations on the safety of the cell-based products are successful, and once commercialization has begun, the FDA will initiate inspections for the production process under its exclusive jurisdiction for cell-based seafood.
This means that the cell-based seafood producers must also ensure that Sanitation Control Procedures and Good Manufacturing Practices are in place for the production. Producers must also develop a science-based risk assessment analysis to control biological, chemical, and physical hazards associated with the type of seafood and processing method.
Labeling of the Cell-based Food Products
Currently, cell-based seafood products have no recommendations or specific labeling requirements. To establish a guideline for labeling cell-based seafood, the FDA published a “Request for Information” in October 2020 to collect input on “the labeling of foods comprising or containing cultured seafood cells” and to understand what types of actions the agency should take to ensure that these foods are properly labeled (FAO and WHO 2023). In the “Request for Information” the agency solicited comments on questions such as 1) which terms are needed to discern cell-based products from others, 2) which terms should be in the product name of a food containing animal cells, 3) which terms could be potentially misleading if names refer to the form of a meat or poultry product (e.g., fillet, steak), and 4) which names might have a negative impact on consumers and seafood industry.
Food Safety Considerations and Potential Hazards at Various Stages of Production
With the growing interest in alternative seafood production, most research studies have focused on the manufacturing methods used in cell-based food production. However, further research is necessary to properly identify and control associated potential food safety hazards (Hadi and Brightwell 2021). This section will briefly review some of the potential hazards associated with each step of cell-based seafood processing.
Potential Hazards Related to Cell Sourcing, Cell Isolation, and Cell Storage
Three primary types of food hazards are biological, chemical, and physical. Biological hazards result from the presence of harmful microorganisms like bacteria, viruses, parasites, or fungi that can cause foodborne illnesses when consumed. Chemical hazards might contaminate food either through natural sources, such as naturally occurring toxins found in certain seafoods; artificial compounds, such as cleaning agents; or excessive natural or artificial food additives. Physical hazards include foreign objects, such as glass, metal, plastic, or any other physical matter, which can accidentally get in food and potentially cause illness or injury to the consumer (SFDPH n.d.).
Bacteria and viruses are the most common cause of foodborne diseases (HHS n.d.). Pathogenic Mycoplasma species, which are the smallest known cell at about 0.1 micron (µm) in diameter, are of particular concern for cell-based seafood production. Up to 35% of the contamination seen in cell lines is attributed to this bacterium (Nikfarjam and Farzaneh 2012). Mycoplasma infections are persistent and usually difficult to detect, diagnose, and cure. Additionally, these bacteria do not grow fast, which delays the detection process. Another major concern related to Mycoplasma is their small size, which enables them to pass most of the filters that are used for cell culture, hence causing contamination (Razin 1996). If not performed correctly, activities related to cell sourcing, isolation, and storage increase the chance of microbial and toxicological contamination within the process. This may happen if the biopsy comes from a source animal that is infected with foodborne pathogens. Pathogens might exist in the biopsied tissues and potentially transfer to the final product, posing a hazard when consumed (FAO and WHO 2023). If detected in early stages, antibiotics can eradicate bacterial contamination. However, once the bacteria enter the log phase of growth, the contamination is not easily treatable, and discarding the culture is recommended (UNC n.d.). For these reasons, the use of antibiotics has become a common practice applied at the initial stages of the cell culture process.
Sourcing the cell lines from quality-controlled cell banks would also decrease the chances of having contaminated cells. Chemical hazards also present a challenge to cell-based seafood production. Some examples of chemical hazards relevant to cell line storage are cryoprotectants, such as dimethyl sulfoxide (DMSO). These cryoprotectants are routinely used for low temperature storage of cell lines, though if misused can have adverse toxicological effects to the consumer. To have a safe final product, the antibiotics and cryoprotectants must be diluted enough or washed out of the process to eliminate or reduce hazards (FAO 2022).
Potential Hazards Related to Cell Culture
Cell culture media is a liquid or gel, composed of vitamins, glucose, necessary amino acids, and inorganic salts, as well as hormones and growth factors. Media can be completely natural or synthetic, and it is used to provide cells with sufficient nutrients and other components necessary for proliferation and differentiation (FAO 2022). The main potential hazard related to cell culture media is the use of animal serum such as fetal bovine serum (FBS). This media could introduce infectious disease agents such as prions into the cell culture (Hadi and Brightwell 2021). Prions are a type of protein that affects the natural foldings of brain proteins, causing them to take on an abnormal shape, and results in prion diseases (Belay and Schonberger 2005). It is suggested that sufficient heat treatment might reduce the chance of contamination. The use of non-animal-derived serum can help with keeping the process contaminant-free (FAO 2022). However, further investigation is required to confirm the effectiveness and functionality of serum-free media. From an economic standpoint, use of serum-free media is not feasible at the industrial scale yet, due to the higher price compared to serum-based media (Hadi and Brightwell 2021). As mentioned earlier, mycoplasma infection must be taken seriously since most species are resistant to antibiotics, and their proliferation rate is remarkably high, thus they are harder to eliminate (Drexler and Uphoff 2002). Maintaining a sterile environment is a key factor not only to keep the cell culture from being contaminated, but also to properly follow good hygiene practices (GHPs), which reduces the need for antibiotic use.
Potential Hazards Related to Scaffolding
Scaffolds are 3D porous structures that support cell attachment and growth and act as a template for tissue formation. This allows for varied structure in the final product that is similar to natural meat in texture and appearance (Seah et al. 2022). It is important to make scaffolds from edible material to ensure they are safe for consumption. The safety of the scaffolds must be assessed before use to ensure they meet food-grade specifications. If scaffolds are made from non-edible material, care must be taken to completely remove them before they reach the consumer, though the use of such scaffolds is gradually being phased out. Scaffolds are often synthesized using chemical crosslinking agents which human tissues can be highly sensitive to. Thus, there is a growing preference for the use of safer crosslinking agents or, alternatively, the elimination of toxic components through flushing and rigorous testing, both to make certain of their absence in the final product (Ong et al. 2021).
Potential Hazards Related to the Harvesting of Cells
During cell harvesting, there are chemical and biological hazards that must be taken into consideration, such as the presence of media residues, buffers, biological contaminants, and other growth by-products. Some of these components can be washed out or reduced by filtration or heat treatments; however, if any of these components or their residues are left in the system, they can become part of the final food product and potentially affect human health. Depending on the harvesting methods used, there is a potential for microbial contamination to be introduced. Pathogenic agents could get into the process due to the use of unhygienic equipment, operators, air, and so forth. Following relevant good practices such as GHPs, proper testing for contaminants and residues, use of food safe components, and conducting food safety assessments can all help minimize the hazards (FAO and WHO 2023).
Potential Hazards Related to Biomanufacturing Cells into Seafood Products
After the harvesting stage, cultivated cells undergo specific modifications and are formulated into commercial food products. At this stage of the process, additives such as flavoring agents or preservatives can be added to enhance the taste, texture, color, and shelf life. The addition of any substances must be evaluated for toxicity and allergenicity prior to use in the process. Also, during this stage of production, following GHPs would decrease the chance of microbiological contamination (FAO 2022). Figure 1 summarizes the potential food safety hazards that can be introduced at various production steps.
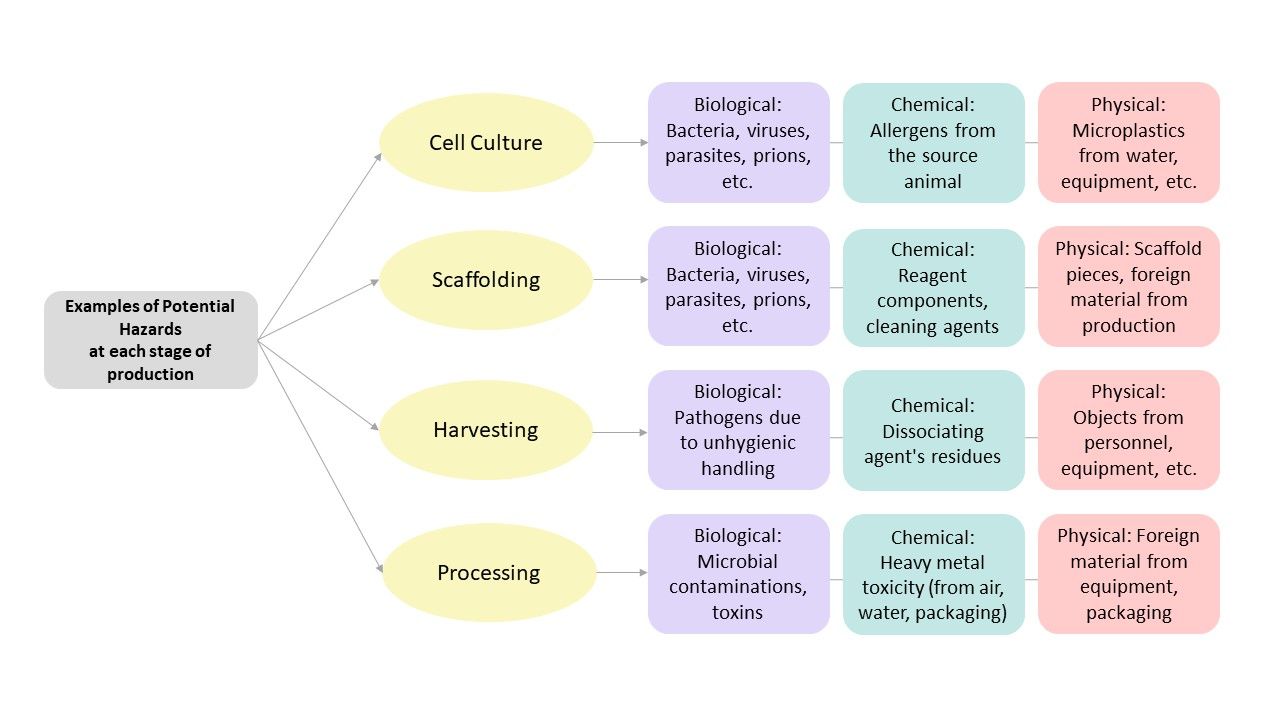
Credit: Rose Omidvar and Razieh Farzad, UF/IFAS
Available Regulations Relevant to Cell-based Seafood Production
Cell–based seafood production regulation varies from country to country. In the United States, there are regulations set by the FDA (2019) for all animal species from which the food or cells are sourced. The FDA’s Center for Veterinary Medicine (CVM) has provided guidelines entitled “Current Good Manufacturing Practices for Animal Cells, Tissues, and Cell and Tissue-Based Products” (ACTPs). Companies must follow these guidelines to ensure safety of the product, species identity, and quality characteristics which they claim. The guidance provides information on the methods, facilities, and controls used for manufacturing ACTPs. These include steps such as recovery, processing, storage, labeling, packaging, and distribution. Cell-based seafood producers should be aware of the biological and chemical by-products and the waste produced during production. Knowing the hazards will aid producers in following the specific regulations that apply to them, such as environmental legislation set by the Environmental Protection Agency.
Conclusion
With the recent research developments in cell-based food technology, the possibility of having cell-based seafood products in the near future is high. As these products come closer to market, regulations must be set to ensure the processes used to produce cell-based products are safe. The FDA and the USDA-FSIS are working with manufacturers to ensure these products meet all applicable regulatory requirements. Ultimately, the manufacturers are responsible for following the guidelines and must be aware of the risks and hazards that would affect the safety of these products.
References
Belay, E. D., and L. B. Schonberger. 2005. “The Public Health Impact of Prion Diseases.” Annual Review of Public Health 26: 191–212. https://doi.org/10.1146/annurev.publhealth.26.021304.144536
Drexler, H. G., and C. C. Uphoff. 2002. “Mycoplasma Contamination of Cell Cultures: Incidence, Sources, Effects, Detection, Elimination, Prevention.” Cytotechnology 39: 75–90. https://link.springer.com/article/10.1023/A:1022913015916
Food and Agriculture Organization (FAO). 2022. Food Safety Aspects of Cell-based Food – Background Document Two: Generic Production Process. Rome. https://doi.org/10.4060/cc2502en
Food and Agriculture Organization (FAO) and World Health Organization (WHO). 2023. Food Safety Aspects of Cell-based Food. Rome, Italy. https://doi.org/10.4060/cc4855en
Hadi, J., and G. Brightwell. 2021. “Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein.” Foods 10 (6): 1226. https://doi.org/10.3390/foods10061226
Nikfarjam, L., and P. Farzaneh. 2012. “Prevention and Detection of Mycoplasma Contamination in Cell Culture.” Cell Journal (Yakhteh) 13 (4): 203–212. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3584481/
Ong, K. J., J. Johnston, I. Datar, V. Sewalt, D. Holmes, and J. A. Shatkin. 2021. “Food Safety Considerations and Research Priorities for the Cultured Meat and Seafood Industry.” Comprehensive Reviews in Food Science and Food Safety 20 (6): 5421–5448. https://doi.org/10.1111/1541-4337.12853
Razin, S. 1996. “Mycoplasmas.” In Medical Microbiology, edited by S. Baron. 4th edition. Galveston (TX): University of Texas Medical Branch at Galveston. Chapter 37. https://www.ncbi.nlm.nih.gov/books/NBK7637/
San Francisco City and County Department of Public Health (SFDPH). n.d. “The Three Hazards to Food.” Environmental Health Section. Consumer Protection-Food Safety Program.” Accessed on June 28, 2024. https://www.sfdph.org/dph/files/EHSdocs/ehsPublsdocs/foodsafetyfacts/food_hazards.pdf
Seah, J. S. H., S. Singh, L. P. Tan, and D. Choudhury, D. 2022. “Scaffolds for the Manufacture of Cultured Meat.” Critical Reviews in Biotechnology 42 (2): 311–323. https://doi.org/10.1080/07388551.2021.1931803
UNC School of Medicine. n.d. “Bacteria.” Lineberger Comprehensive Cancer Center. Accessed on June 28, 2024. https://unclineberger.org/tissueculture/contaminant/bacteriacontam/
U.S. Department of Health and Human Services (HHS). n.d. “Bacteria and Viruses.” Last reviewed September 19, 2023. https://www.foodsafety.gov/food-poisoning/bacteria-and-viruses
U. S. Food and Drug Administration (FDA). 2019. “Formal Agreement Between FDA and USDA Regarding Oversight of Human Food Produced Using Animal Cell Technology Derived from Cell Lines of USDA-amenable Species.” https://www.fda.gov/food/domestic-interagency-agreements-food-expired/formal-agreement-between-fda-and-usda-regarding-oversight-human-food-produced-using-animal-cell