
Credit: Michele Wood UF/IFAS Communications
Contents
- Stonefruits in Florida—Introduction
- Stonefruit Quality—Evaluation and Measurement
- Stonefruit Cultivars for Florida
- Postharvest Physiology of Stonefruits
- Stonefruit Harvest Operations
- Stonefruit Packing Operations
- Controlling Postharvest Decays of Stonefruits Grown in Florida
- Managing the Postharvest Environment – Best Practices for Stonefruit Precooling, Storage, and Shipping
- Postharvest Stonefruit Defects and Disorders
- Distribution, Marketing, and Consumer Handling of Florida Stonefruits
1. Stonefruits in Florida – Introduction
Ali Sarkhosh and Mercy A. Olmstead
Growers of many high-value horticultural commodities have taken advantage of the subtropical climate in Florida to become the earliest domestic source of several fruits and vegetables during the winter and spring months, leading to profitable endeavors. Currently, Florida produces some of the earliest commercial-quality stonefruit (peach, nectarine, and plum) in North America. The low-chill cultivars of stonefruit developed by the Stonefruit Breeding Program operated by the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) do not require as much winter chilling to produce fruit as traditional cultivars that are grown farther north in the United States. These peach, nectarine, and plum cultivars, whose names all begin with “UF,” allow growers to produce an early-ripening, high-quality fruit with excellent flavor. For example, ‘UFSun’ (Figure 1-1) and ‘UFBest’ peaches, which are adapted to central- and south-central Florida, have a fruit development period (FDP) from bloom to harvest of approximately 80 days. When grown in south-central Florida, they ripen in early April and are among the first commercial peaches to harvest in North America. ‘UFSun’ and ‘UFBest’ are followed closely in ripening sequence by several other peach and nectarine cultivars adapted for south-central to northern Florida. In 2017, the Florida peach industry produced over 3,000 tons of fruit from an estimated 2,000 acres (Harders et al. 2017).

Credit: Ali Sarkhosh, UF/IFAS
From North to South: Breeding Program History
Although there were about 4,000 acres of stonefruit in Florida in the late 1970s and early 1980s, predominantly located in northern Florida, a combination of market factors and severe weather events led to the overall decline of the peach industry in the southeastern United States. A major contributor to the decline of peach acreage was the lack of frost-protection systems during the 1980–89 freezes, which led to the removal of several thousand acres of peaches. However, UF/IFAS continued the Stonefruit Breeding Program and released several new cultivars, many of which are grown worldwide.
Dr. Ralph Sharpe began the UF/IFAS's Stonefruit Breeding Program in 1952 with the goal of taking advantage of Florida's climate, land, and market window to produce some of the earliest fruit in the United States (Sherman et al. 1996). Florida's environment is unique in the continental United States. Our climate is subtropical and monsoonal, which means that it affords stonefruit trees a relatively long period of growth postharvest. Producers have capitalized on the unique benefits of the Florida climate to deliver the earliest domestic production of high-value commodities such as vegetables, tomatoes and peppers, and fruit, like strawberries and blueberries.
Peaches and nectarines (Prunus persica L. Batsch.) are related to species of Prunus native to China (Bassi and Monet 2008). Peaches, nectarines, and other stonefruit originate from temperate climates that require exposure to cold temperatures for flowering and resumption of vegetative growth in the spring. The amount of cold required for growth is measured in chill units. A chill unit is a period of time between 32 °F and 45 °F (0 °C –7 °C) that accumulates through the winter months (Richardson et al. 1974). A temperate fruit crop requires a certain number of chill units to bloom and bear fruit. However, in Florida, which is considered a subtropical environment, chill units do not accumulate to the extent that they do in temperate climates. Thus, the germplasm used in the UF/IFAS breeding program had to include low-chill sources of material, including some from southern China. Of these importations of germplasm, the most important was that from Okinawa, Japan. This plant material provides the backbone low-chill cultivars in the UF/IFAS breeding program.
Dr. Wayne Sherman succeeded Dr. Sharpe as the UF/IFAS stonefruit breeder in January of 1966. Dr. Sherman was responsible for several improvements that increased the efficiency of the breeding program and reduced the time from making a cross to the release of a new cultivar. He developed the fruiting nursery, correlated early ripening with the appearance of red leaves, and began the use of the non-melting-flesh type for fresh fruit production.
Peaches are typically available in two different flesh types, melting and non-melting. Melting-flesh peaches undergo a rapid softening of the flesh as the fruit ripen. Historically this type of fruit has been used for fresh fruit production. Non-melting-flesh peaches are characterized by a firm flesh phenotype that softens slowly, resulting from a natural mutation that was identified hundreds of years ago. Non-melting-flesh peaches are primarily used for processing. Melting-flesh cultivars typically do not ship as well as they approach physiological ripeness because of their tendency to bruise easily, and thus are harvested much earlier to withstand transport within the marketing chain. With an early harvest, sugar levels (often measured as °Brix), flavor, and color may not be as well developed. Fruit quality is an important consideration for consumers that make return purchases (Crisosto 2002); however, it can be difficult to achieve in early-ripening cultivars (Sharpe et al. 1954).
In order to develop peach cultivars that could be harvested when “physiologically mature” so as to achieve higher fruit quality, non-melting-flesh genes in plant material from Mexico, North Carolina, and Brazil were brought into the UF/IFAS breeding program. These genes were used to breed non-melting-flesh peach cultivars that can be harvested at a more mature (ripe) stage with a firm texture, greater flavor, increased color development, and higher sugar content. Recent research has shown that non-melting fruit must be harvested later than melting-flesh cultivars, to develop optimum flavor profiles associated with high-quality fruit, allowing fruit to stay on the tree until physiologically mature (Kao et al. 2008). Growers and fruit marketers have taken advantage of this fruit quality trait to promote a true “tree-ripe” product.
Chilling Requirement
Stonefruits are temperate-zone tree fruits that require a minimum amount of accumulated cold temperature exposure (between 32°F [0°C] and 45°F [7°C]) to resume normal growth the following spring. This is referred to as the cultivar’s chilling requirement (Richardson et al. 1974; Sherman and Rodriguez-Alcazar 1987; Sharpe et al. 1990). The chilling requirement is usually expressed in chill units (cu). It counts any hour between 32°F–45°F (0°C–7°C), temperatures which, in Florida, occur predominantly from October to the first week of January. Each cultivar has its own characteristic chilling requirement, which partially determines its adaptability to a certain region of the state (Table 1-1, Figure 1-2). The importance of proper site and cultivar selection must be emphasized. For example, ‘Gulfcrest’ (525 cu) is adapted to north Florida (Panhandle region) but would not receive sufficient chilling most winters to grow well in Gainesville. Conversely, if the cultivar’s chilling requirement is too low for the area where it is planted, there is a greater chance of late-winter frosts killing blossoms and young fruit. More peaches and nectarines are lost to frost damage in the southeastern United States than to any other single cause.
Table 1-1. Chilling requirement characteristic of UF/IFAS peach and nectarine cultivars (Sarkhosh et al. 2018a).
![Figure 1-2 Chill units accumulated (between 32°F [0°C] and 45°F [7°C]) through February 10 in 75% of winters.](/image/HS1459/Dgc11qlc51/Iywz3abel8/Iywz3abel8-2048.webp)
Credit: UF/IFAS
Plums could be a potential crop for growers and homeowners in Florida and other mild winter areas throughout the Gulf coast, but many plum cultivars from the western United States will not consistently perform well enough in Florida to produce fruit. However, the UF/IFAS Stonefruit Breeding Program has developed cultivars that improve the potential for growing plums in Florida and other mild winter areas with high disease pressure. These cultivars are recommended for trial in Florida. The names of all the UF/IFAS plum cultivars begin with the prefix ‘Gulf’. These cultivars are Japanese type plums (Prunus salicina Lindl.) and have resistance to plum leaf scald (Xylella fastidiosa) and bacterial spot (Xanthomonas campestris). Fruit size is satisfactory (about 1½ to 2 inches in diameter) with good fruit quality. They ripen in early to late May, approximately 2 weeks before plums from other areas arrive in the marketplace (Sarkhosh et al. 2018b).
Marketing Situation
The incentives for growing peaches and nectarines in Florida are 1) production and marketing of fresh Florida peaches and nectarines before central Georgia, South Carolina, and California enter the market, and 2) production of quality fruit when there is almost no other quality fresh fruit of any kind available (Figure 1-3). Peaches and nectarines can be produced continuously from late March until late May or early June in Florida, depending on the weather. As precipitation increases during the summer months, disease and pest problems on peaches and nectarines also increase. Later-maturing cultivars have little or no potential in Florida because of increased pest and disease pressure and increased market competition.
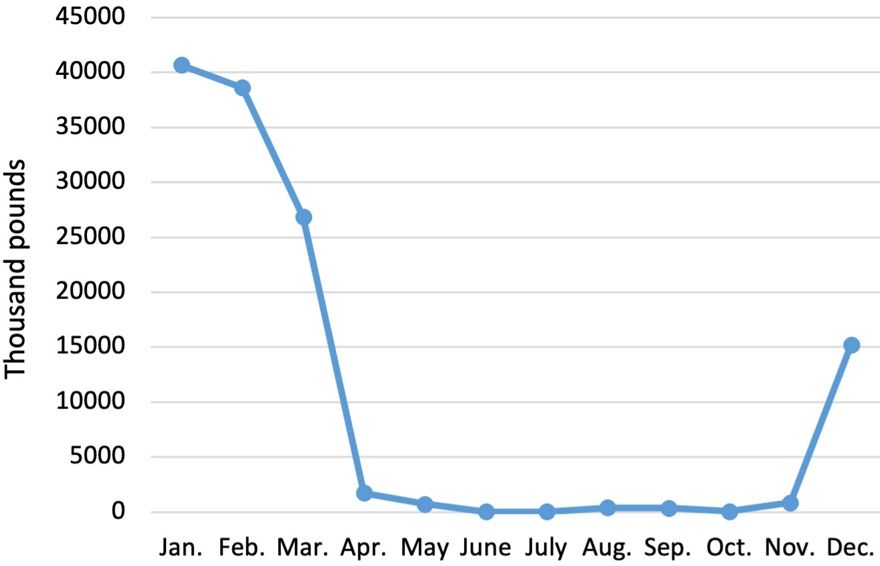
Credit: Economic Research Service, USDA-ARS
To market high-quality peaches, it is necessary to harvest each cultivar three to four times at 2-day intervals in order to obtain fruit that are tree ripe. When harvested at this stage, fruit have the highest potential for flavor. For long-distance shipment, fruit must be carefully handled, graded, sized, cooled, and packed. This requires a sizable investment in a packinghouse, which is not likely to prove economically feasible with much less than 100–150 acres. However, marketing alternatives for smaller growers include joining a grower cooperative; making direct sales to grocery stores or produce markets; and opting for U-pick, farmers’ markets, or roadside stand operations. It is important to grow at least two to three cultivars that ripen in succession to ensure a steady flow of ripe fruit. This longer harvest period helps with the marketing and efficient use of harvest labor and packing facilities.
Commercial peach and nectarine operations in the southeastern United States can have pre-harvest production costs of about $4,000 per acre (Morgan et al. 2010; M. Olmstead personal communication). For more information about production cost and profitability of peach orchards, please refer to the publication Establishment and Production Costs for Peach Orchards in Florida: Enterprise Budget and Profitability Analysis (Singerman et al. 2017). Establishment costs vary depending on the grower’s situation. Costs will be lower, for instance if the land is already purchased and irrigation is available from a previous crop. Because marketing costs for commercial orchards are relatively high, U-pick operations have the potential to greatly reduce total costs. Estimated returns (gross) to peach growers in Florida are approximately three times greater than those of growers in surrounding southeastern states (United States Agricultural Statistics Board 2011).
Labor
Peach and nectarine production requires high levels of seasonal hand labor. Commercial growers hire labor for dormant and summer pruning, fruit thinning, and harvesting. Family operations often supply their own labor. Timing of labor operations is especially critical; often a delay of a few days in harvesting can result in profit loss. Currently, the greatest cost of peach production in Florida is hand thinning and manual pruning. These practices together require around 150 hours per acre depending on tree size and fruit set, resulting in an estimated cost of approximately $2,000/ acre per year.
Planting Guidelines
Peaches and nectarines should only be planted on sites with excellent air drainage to reduce the risk of frost damage to flowers. Cold air is denser and sinks to the lowest spot in the orchard, especially on nights with radiation freezes. Radiation freezes occur on clear nights with calm wind conditions, when warm air rises as the cold air sinks (Figure 1-4). An advective freeze is dominated by windy conditions and often results in severe damage to the flowers and potential fruit. On a good site, select cultivars with chilling requirements slightly less than the average chilling received in that location. This helps ensure adequate chilling during unusually warm winters; if the site has excellent air drainage, the risks of flower damage from spring freezes will be minimal during normal and high-chill years.
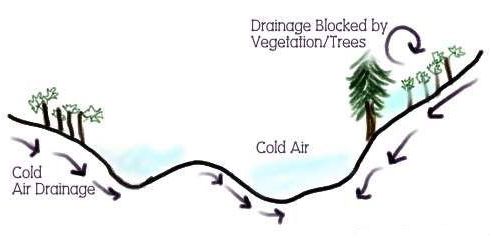
Credit: M. Olmstead, UF/IFAS
Site selection and cultivar choice rank as the two most important factors in successful peach and nectarine growing. When selecting a site, avoid low areas characterized by late spring frosts. Even in central Florida, freezes can occur throughout February and early March in cold locations; thus, sites with good air drainage are essential for reliable production. Planting an orchard with the proper site and cultivar will allow growers to maximize their establishment efficiency with the minimum number of replanted trees.
Peaches and nectarines can be grown on a wide variety of soils, provided the upper 4–6 feet of soil is well-drained. Avoid “hardpan” soils unless an excellent system of subsoil drainage tiles or a bedded system is installed. Irrigation is required throughout the trees’ growth and fruit development periods to obtain acceptable fruit size and yields. A properly designed overhead sprinkler irrigation system has the added advantage of protecting flowers and young fruit from late winter and spring freezes. For low-volume irrigation systems, micro-sprinklers are preferred to drip emitters. To avoid spraying seedlings/trees, a truck with water micro-sprinklers with 240 to 300 degrees spraying pattern is recommended.
June-budded trees that are 2 ½–4 feet high are a good size to plant during the fall season, while larger sizes are more difficult to handle and expensive. Normal tree spacing is 15 x 20 feet, or 145 trees per acre. On lighter soils, higher density plantings have been satisfactory (Table 1-2). All Florida peach and nectarine cultivars are self-fruitful and should be planted in solid blocks for easier spraying and harvesting.
Table 1-2. Various tree and row spacing for orchard establishment.
Cultural Program
Growing peaches and nectarines requires a high level of skilled management, which includes pruning, fruit thinning, harvesting, and scouting for pests and diseases (Figure 1-5). Peach and nectarine trees are susceptible to a number of health challenges, including diseases, insects, and nematodes. Root-knot nematodes (Meloidogyne incognita and M. javanica) are common in Florida soils and can weaken peach and nectarine trees. In addition, peach root-knot nematode, M. floridensis (Handoo et al. 2004), can infect common peach rootstocks such as ‘Nemaguard’ and ‘Okinawa’. Therefore, only M. floridensis root-knot nematode-resistant rootstocks such as ‘Flordaguard’ or ‘MP-29’ should be used in Florida (Sherman et al. 1991; Sarkhosh et al. 2018c). An integrated pest management program must be followed to ensure good fruit quality. Although some diseases and insects can be severe, they can usually be controlled with a proper pest management program. A disease and pest management guide is available online at the UF/IFAS stonefruit website (https://hos.ifas.ufl.edu/stonefruit/).

Credit: Ali Sarkhosh, UF/IFAS
Additional Reading
Bassi, D., and R. Monet. 2008. “Botany and Taxonomy.” In The Peach: Botany, Production and Uses, edited by Layne and Bassi, 1–36. Wallingford, U.K.: CAB International Press. https://doi.org/10.1079/9781845933869.0001
Handoo, Z. A., A. P. Nyczepir, D. Esmenjaud, J. G. van der Beek, P. Castagnone-Sereno, L. K. Carta, A. M. Skantar, and J. A. Higgins. 2004. “Morphological, Molecular, and Differential-Host Characterization of Meloidogyne floridensis n. Sp. (Nematoda: Meloidogynidae), a Root-Knot Nematode Parasitizing Peach in Florida.” Journal of Nematology 36 (1): 20–35.
Harders, K., J. Rumble, T. Bradley, L. House, and A. Sandra. 2016. Consumer Peach Purchasing Survey. PIE2016/17-02. Gainesville, FL: University of Florida/IFAS Center for Public Issues Education. https://www.piecenter.com/wp-content/uploads/2015/09/Peach-Report_FINAL.pdf
Kao, M-W., J. G. Williamson, and J. K. Brecht. 2008. “Optimum Harvest Maturity for Extended Postharvest Quality of Melting and Non-Melting Flesh Subtropical Peach Varieties. HortScience 43:1149–1150.
Morgan, K., J. Braswell, F. Matta, D. Ingram, and B. Layton. 2010. Peach - Fruit and Nut Planning Budgets. Starkeville: Mississippi State University.
Richardson, E. A., S. D. Seeley, and D. R. Walker. 1974. “A Model for Estimating the Completion of Rest for 'Redhaven' and 'Elberta' Peach Trees.” HortScience 9:331–332. https://doi.org/10.21273/HORTSCI.9.4.331
Sarkhosh, A., M. Olmstead, J. Chaparro, P. Andersen, and J. Williamson. 2018a. “Florida Peach and Nectarine Varieties: Cir1159/MG374, Rev. 10/2018.” EDIS 2018:17. https://doi.org/10.32473/edis-mg374-2018
Sarkhosh, A., M. Olmstead, P. C. Miller, P. Andersen, and J. Williamson. 2018b. “Growing Plums in Florida: HS895/HS250, Rev. 9/2018.” EDIS 2018:13. https://doi.org/10.32473/edis-hs250-2018
Sarkhosh, A., M. Olmstead, J. Chaparro, and T. Beckman. 2018c. “Rootstocks for Florida Stone Fruit: HS1110/HS366, Rev. 10/2018.” EDIS 2018 (6). https://doi.org/10.32473/edis-hs366-2018
Sharpe, R. H., T. E. Webb, and H. W. Lundy. 1954. “Peach Variety Tests.” Proceedings of the Florida State Horticultural Society 67:245–246.
Sharpe, R. H., W. B. Sherman, and J. D. Martsolf. 1990. “Peach Varieties in Florida and Their Chilling Requirements.” Acta Horticulturae 279:191–197.
Sherman, W. B., and J. Rodriguez-Alcazar. 1987. “Breeding of Low-Chill Peach and Nectarines for Mild Winters.” HortScience 22:1233–1236.
Sherman, W. B., P. M. Lyrene, and R. H. Sharpe. 1991. “‘Flordaguard’ Peach Rootstock.” HortScience 26 (4): 427–428.
Sherman, W. B., P. M. Lyrene, and R. H. Sharpe. 1996. “Low-Chill Peach and Nectarine Breeding at the University of Florida.” Proceedings of the Florida State Horticultural Society 109:222–223.
Singerman, A., M. Burani-Arouca, and M. Olmstead. 2017. “Establishment and Production Costs for Peach Orchards in Florida: Enterprise Budget and Profitability Analysis: FE1016, 7/2017.” EDIS 2017 (4). https://doi.org/10.32473/edis-fe1016-2017
United States Agricultural Statistics Board. 2011. “Noncitrus Fruits and Nuts 2010 Summary.” National Agricultural Statistics Service (NASS), Agricultural Statistics Board, U.S. Dept. of Agriculture. http://usda.mannlib.cornell.edu/MannUsda/viewDocumentInfo.do?documentID=1113.
2. Stonefruit Quality – Evaluation and Measurement
Jeffrey K. Brecht and Mark A. Ritenour
Producing high-quality stonefruit requires the grower to maintain the trees in an optimum range of nitrogen and water availability; use proper pruning to optimize light penetration into the canopy, which improves fruit size, color, and sugar content; and conduct fruit thinning to achieve maximum size and sugar content in the remaining fruit.
However, the most important factor influencing stonefruit quality is whether the fruit are fully mature at harvest. Short of allowing the fruit to fully ripen on the tree, at least letting the fruit initiate ripening prior to harvesting them results in the best quality fruit for the consumer. Stonefruit that have not fully developed on the tree are more prone to postharvest water loss (shriveling); chilling injury (internal breakdown); and abrasion damage. They are also of inferior quality when ripe. Fruit that are too ripe at harvest, on the other hand, are susceptible to decay and many riper fruit will never make it through packing and transport, being more prone to bruising from impacts and compression. However, most of the stonefruit cultivars from the Stonefruit Breeding Program operated by the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) have had the non-melting-flesh trait incorporated into them, which allows the fruit to maintain greater firmness as they begin to ripen. Cultivars with the non-melting-flesh trait may be allowed to begin ripening before harvest without becoming too soft to tolerate harvesting, packinghouse handling, and transportation stresses.
United States Standards for Quality of Fresh-Market Peaches. The following section lists the most basic standards that most stonefruits in the marketplace are required to meet. (The first four grades of peaches are a close paraphrase of the language on the USDA peach grades and standards webpage, here: https://www.ams.usda.gov/grades-standards/peach-grades-and-standards.) However, many retailers have more stringent standards. The US standards are primarily based on the visual appearance of the fruit with very small tolerances for soft or overripe fruit.
U.S. Fancy consists of peaches of one cultivar that are mature but not soft or overripe, well-formed, and free from decay, bacterial spot, unhealed cuts, growth cracks, hail injury, scab, scale, split pits, worms, worm holes, leaf or limb rub injury; and free from damage caused by bruises, dirt or other foreign material, other disease, insects or mechanical or other means. This grade requires that the peaches have at least 1/3 of their surface showing blushed pink or red color and that at least 90% of them meet this color standard. U.S. Fancy has a 2% allowance, or tolerance, for soft or overripe fruit at destination.
U.S. No. 1 peaches meet the same standards as U.S. Fancy except for not having a color standard; the fruit should be mature with a 2% allowance for soft or overripe peaches at destination.
U.S. Extra No. 1 peaches meet the requirements of U.S. No. 1 grade, provided that at least 50% of the fruit have at least 1/4 of their surface showing blushed pink or red color. As with U.S. Fancy and U.S. No. 1, no more than 2% of the fruit are allowed to be soft or overripe at destination.
U.S. No. 2 consists of peaches of one cultivar that are mature, but not soft or overripe, not badly misshapen, and free from decay, unhealed cuts, worms, worm holes; and free from damage caused by bruises, dirt or other foreign material, bacterial spot, scab, scale, hail injury, leaf or limb rubs, split pits, other disease, insects or mechanical or other means. There are no color standards for this grade. As with all USDA grades, the tolerance for soft or overripe fruit at destination is only 2%.
U.S. Mature is a standard that covers all U.S. No. 1 peaches, stipulating that the fruit are mature enough to complete the ripening process without additional ethylene exposure. There is a non-severe open suture tolerance of 25%.

Credit: undefined
California Well Matured. This is another standard that is commonly seen in the marketplace. These fruit must be mature enough to complete the ripening process without additional ethylene exposure. The over-blush is usually 90% of total for a given cultivar. Ninety percent of the lot must meet the color standard. There is a non-severe open suture tolerance of 25%.
The quality of stonefruits is related to their highly appreciated and desirable appearance, texture, taste, aroma, and nutritive value. These same aspects of stonefruit quality are used to determine the ripeness and desirability of stonefruit.
Recommended Practices for Evaluating and Measuring Stonefruit Quality
Appearance: Fruit Size, Shape, Defects, and Color
Size and shape of stonefruits are important features for successful marketing. It is possible for pickers and packers to subjectively determine if peaches and other stonefruits are within certain size ranges and that the fruit shape is normal and uniform. Accurate size measurement requires the use of calipers or sizing rings. Weight is also a fairly accurate measure of fruit size if the fruit are uniformly shaped.

Credit: Mark A. Ritenour, UF/IFAS
Besides misshapen fruit, defects may include such things as russeting, scarring, split pits, insect evidence, and decay that can be avoided at harvest or graded out on the packingline. Defects also include damage occurring postharvest, such as abrasions and bruising, peel “inking,” and decay that, with the help of visual aids, can be identified and subjectively scored based on incidence and severity.
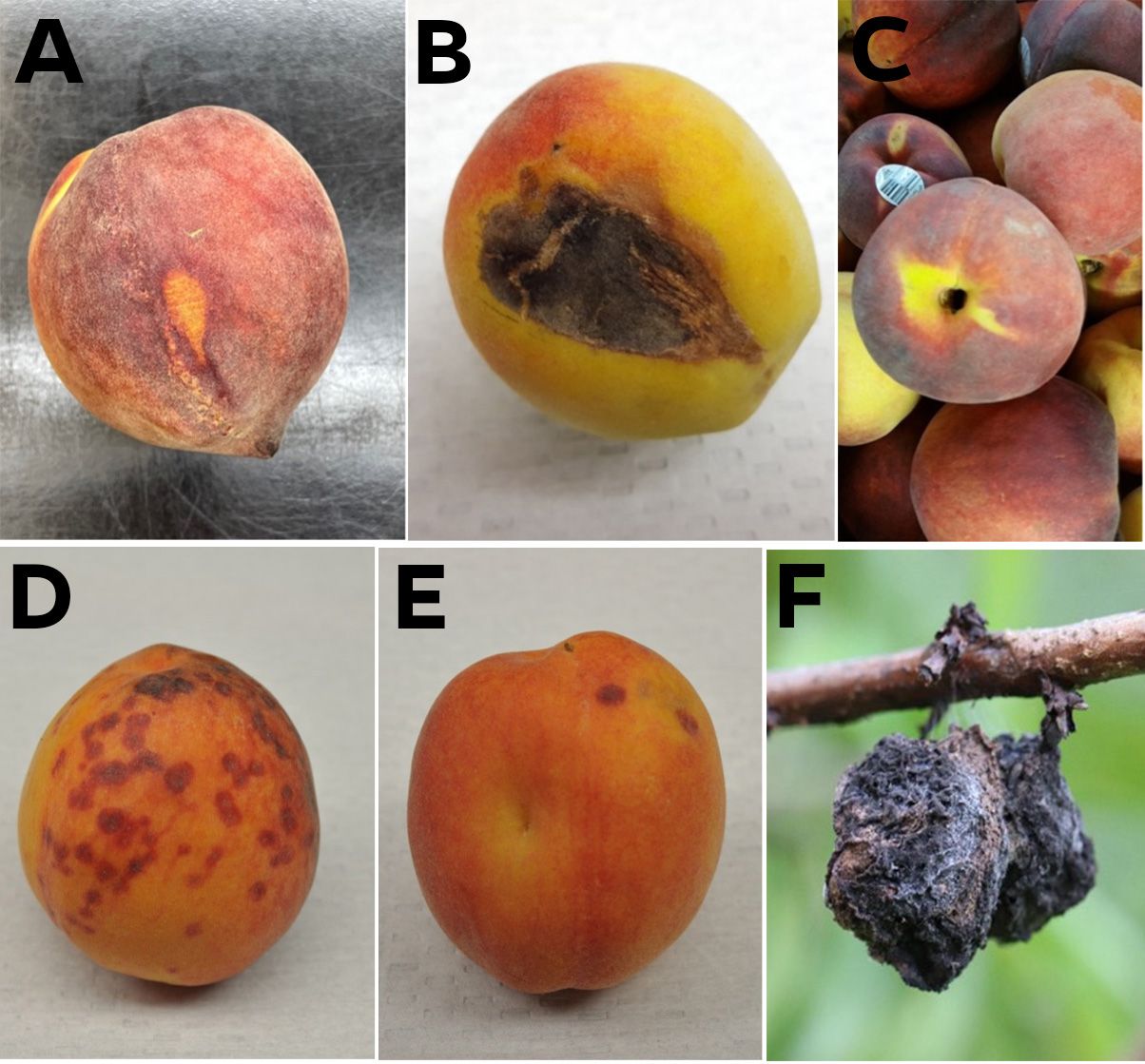
Credit: A, B, C, D, and E, Mark A. Ritenour, UF/IFAS; F, Phil Harmon, UF/IFAS
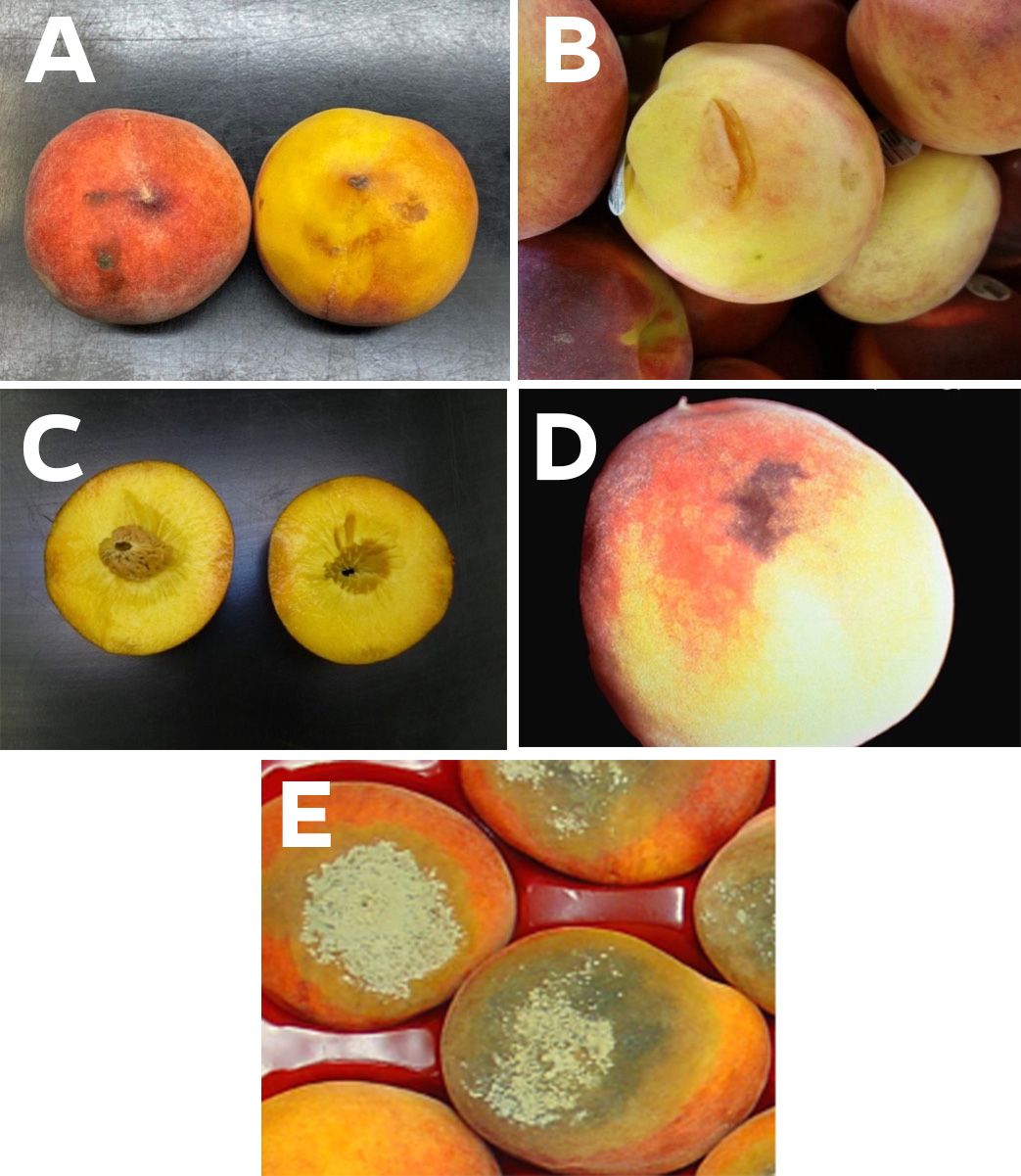
Credit: A, B, C, Mark A. Ritenour, UF/IFAS; D, Carlos Crisosto, UC Postharvest Technology Center; E, Yuru Chang, UF/IFAS
Some types of stonefruits, such as apricots, plums, and processing peaches, may have more or less uniform color on the fruit surface, or, as in fresh-market peaches, there may be a combination of colors. In the latter case, the red color or “blush” is due to exposure to sunlight. While the presence of blush may be important for marketing, it does not have any relationship to ripeness or any other quality aspect of the fruit. More important in terms of quality is the color underlying the blush that changes from green to yellow and sometime orange as peaches and nectarines ripen. This underlying color is called the “ground color” and it is the most important color to which attention should be paid when judging fruit quality and ripeness.

Credit: Jeffrey K. Brecht, UF/IFAS
Color charts and guides have been made for stonefruits in different production areas to assist in evaluating fruit maturity and ripeness. Color charts are usually photographs of fruit at different stages of ripeness. Subjectively scoring color with reference to such a chart can be quite practical and accurate. A popular style of color guide is a ring or color chip with a hole that is meant to be held over the fruit. This design makes matching the fruit and guide colors quite easy.
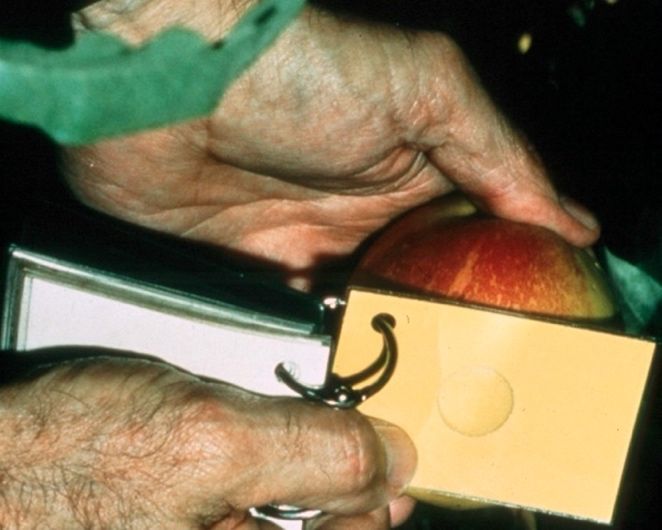
Credit: Carlos Crisosto, UC Postharvest Technology Center
The standard laboratory instrument used to quantitatively measure color is the colorimeter or chromameter. A port on the chromameter is held against the fruit and the reflected light from a flash directed against the fruit is measured and separated into components that represent the lightness to darkness of the color, the hue or shade of color, and the chroma or color purity. The chromameter is very accurate and the measurements very reproducible, but the cost ($9,000 to $10,000) may make it impractical for use outside of a research laboratory.

Credit: Jeffrey K. Brecht, UF/IFAS
Texture: Flesh Firmness and Juiciness or Mealiness
Stonefruit flesh firmness is an important measure of ripeness, and the range and pattern of softening varies widely among the different types of stonefruits as well as among different cultivars of the same type of fruit. This is especially true for peaches, which include cultivars with a number of different genes controlling flesh texture. For Florida peaches, the important distinction is between the traditional melting-flesh cultivars and the newer non-melting-flesh cultivars. Both types are equally firm before they begin to ripen, but melting peaches soften very rapidly during ripening and become extremely soft while non-melting-flesh fruit largely retain their firmness for the first one-third or so of their ripening then soften slowly and never become quite as soft as the melting-flesh fruit.
Fruit juiciness is a desirable quality feature for all types of stonefruits. Lack of juiciness is often called “mealiness,” referring to the dry mouthfeel that reminds people of a mouthful of corn meal. Mealiness is actually the result of abnormal softening in which the fruit cell walls do not break down properly, trapping the liquid in a complex with pectin (the same pectin used to make jams and jellies set up). Mealiness is associated with the chilling injury-related disorder called “internal breakdown.”
Firmness can be judged by squeezing the fruit. With experience, this can be somewhat accurate, especially for softer fruit. Harvesters should lightly depress the blossom end of fruit with their thumb while grasping the whole fruit in their hand in order to judge whether or not to detach it.
A more accurate and repeatable, and therefore more reliable, method for measuring fruit firmness is to use a penetrometer. A penetrometer is used to push a convex-tipped probe against the flesh of the fruit (peel removed) until the tissue can no longer resist the force being applied and fails, allowing the probe to penetrate the flesh. The force that is recorded is called the “bioyield point.” The probe is connected by a spring to a force gauge that shows firmness on a pounds-force or kilogram-force scale. The diameter of the probe affects the reading (a wider probe gives a higher reading); for stonefruit, a 5/16-inch or 8-mm diameter probe is the correct size. It’s important to remove a small patch of peel before measuring firmness with a penetrometer. This is because, as the fruit softens, the peel can eventually become a greater barrier to penetration of the probe than the underlying flesh, resulting in inaccurate measurements.

Credit: Jeffrey K. Brecht, UF/IFAS
Hand-held penetrometers are most common, but various other types have been developed that mount the probe and force gauge in different ways in attempts to reduce operator variability. More advanced types of texture analyzers are motorized to completely remove operator variability and are connected to a computer so that the texture profile as the probe penetrates the flesh can also be recorded.
Taste and Aroma (Flavor): Soluble Solids and Acidity
The flavor of stonefruits is due to unique combinations of taste and aroma. The taste is primarily a product of the sugars and organic acids that are present, while the aroma comes from our perception of a complex array of volatile compounds produced by the fruit.
The main method that is used to get at the taste component of stonefruit flavor is to measure the soluble solids content with a refractometer. The refractometer scale is in degrees Brix and is a measure of the refractive index or the degree to which light is refracted or bent by soluble compounds as it passes through a prism. The temperature of the solution affects the refractive index (solutions expand and become less dense as temperature rises); for sugar solutions, the change is about 0.5 degrees Brix per 10 °F (-12 °C). Better-quality refractometers have built-in temperature compensation. Otherwise, the temperature needs to be measured and a correction made before recording the result.
The Brix measurement is made by taking a few drops of juice that have been expressed from the fruit tissue and placing them on the refractometer prism; the refractometer is then held up to the light and the degrees Brix read from a scale. Digital refractometers are also widely available that do not have to be held to the light and that present the result on an LED readout. Refractometers can be calibrated using distilled water (0 degrees Brix at 68 °F [20 °C]). The juice must be rinsed from the prism and the prism dried with a tissue between measurements.
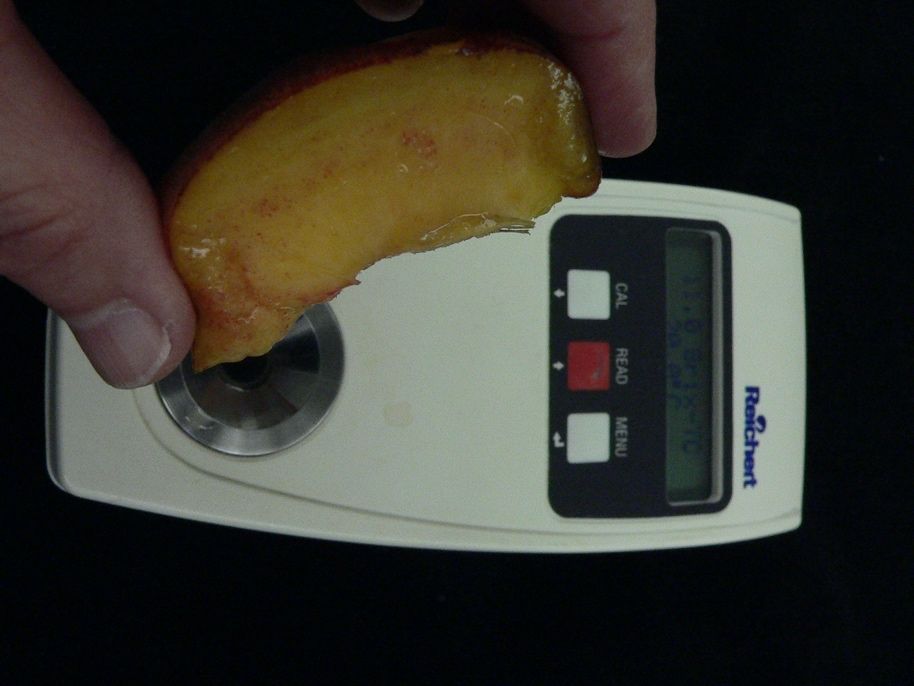
Credit: Jeffrey K. Brecht, UF/IFAS
The major component of soluble solids in fruit juice is the sugars, so Brix readings are often used as a kind of shorthand for sugar content or sweetness. However, other compounds in the juice, such as organic acids, amino acids, phenolics, and soluble pectins, also contribute to the Brix reading.
Fruit acidity is not measured as commonly as soluble solids content, not because it is less important to taste, but because it is less convenient to measure. Until recently, acidity has been measured by titrating a juice sample with a basic solution (usually 0.1 N sodium hydroxide) to an end point of pH 8.2 that is determined either by a pH meter or by the color change of phenolphthalein dye compound. The percent acidity is then calculated using one of three formulas based on whether the predominate acid in the fruit being measured is citric, malic or tartaric acid (note: it is malic acid for stonefruits).
The formula for determining percent titratable acidity from milliliters of 0.1 N sodium hydroxide required to change the juice pH to 8.2 is,
percent acid = [mL NaOH used] X [0.1 N NaOH] X [milliequivalent factor of 0.067 for malic acid] X 100 / grams of juice in sample
This is obviously a laboratory procedure that most producers and handlers don’t care to undertake on a routine basis. However, recently, portable digital acidity meters have become available that are operated very much like a digital refractometer.

Credit: Mark A. Ritenour, UF/IFAS
Taste panels and consumer testing can be useful to determine, in the first case, if there is a flavor difference that can be perceived, and in the second, what consumers like and don’t like about different fruit. Important information about appearance, texture and flavor can be obtained from taste panels and consumer tests, for example, on acceptability prior to introducing a new cultivar, or to determine how harvest maturity affects consumer preference for the ultimate ripe fruit. Methods for conducting taste panels and consumer tests are available from various sources, and there are also companies that specialize in conducting such tests.

Credit: Jeffrey K. Brecht, UF/IFAS
Nutritive Value: Vitamins and Minerals
Stonefruits are an abundant source of ascorbic acid or vitamin C, but the vitamin can be easily lost in fruit that suffer temperature abuse or other kinds of stress. The yellow to orange pigments in stonefruits are carotenoids, which are nutritionally important as antioxidants in our diet and include beta-carotene, also known as pro-vitamin A. Stonefruit also contain another class of antioxidants called phenolics. The phenolics in stonefruit may be red (like the blush on the peel) or colorless. The methods used to measure vitamin C, vitamin A, carotenoids, and phenolics involve fairly sophisticated laboratory procedures and instrumentation that are not practical for producers or handlers. However, service labs will measure these compounds if you wish to include them on Nutrition Facts labeling.
Paying attention to stonefruit quality starts with cultivar selection (Chapter 3) and includes using horticultural practices that promote production of good quality fruit. Careful harvesting and postharvest handling practices (Chapters 5 and 6) reduce physical injuries that lower stonefruit grades. It is important to take steps to minimize chances for decay (Chapter 7) and for contamination and proliferation of microorganisms that endanger food safety (Chapter 8). Rapid cooling to and handling at safe temperatures that do not cause internal breakdown along with proper humidity control to avoid shriveling (Chapter 9) help maintain good quality. Finally, proper ripening practices (Chapter 4) result in the best flavor, increased customer satisfaction, and greater sales.
Additional Reading
Central Valley Postharvest Newsletter. 2008. Vol. 17, No. 2. http://ucanr.edu/datastoreFiles/234-2059.pdf (Contains detailed descriptions of procedures for measuring 1) pH and titratable acidity, 2) fruit firmness, and 3) internal breakdown and soluble solids content.) Crisosto, C. H., F. G. Mitchell, and Scott Johnson. 1995. “Factors in Fresh Market Stone Fruit Quality.” Postharvest News and Information 6 (2): 17N–21N.
Kader, A. A. 2008. “Flavor Quality of Fruits and Vegetables.” Journal of the Science of Food and Agriculture 88:1863–1868. https://doi.org/10.1002/jsfa.3293
Mitcham, E. A., M. Cantwell, and A. Kader. 1996. “Methods for Determining Quality of Fresh Commodities.” UC Davis Perishables Handling Newsletter Issue No. 85. 5 p. (updated 2003) http://ucce.ucdavis.edu/files/datastore/234-49.pdf
United States Dept. of Agriculture, Agricultural Marketing Service. Fresh Market Fruit Grade Standards. http://www.ams.usda.gov/AMSv1.0/freshmarketfruitstandards (standards for apricots, cherries, nectarines, peaches, and plums, along with handbooks and visual aids for inspectors for cherries and peaches).
3. Stonefruit Cultivars for Florida
Ali Sarkhosh, Mercy A. Olmstead, and Jose X. Chaparro
One of the most important decisions taken by a grower is to ensure that a chosen cultivar is adapted to a particular site. Particular peach cultivars destined for commercial production are recommended for each area in order to ensure that the cultivars’ chilling unit requirements will be met in most years. Cultivars with higher chill unit requirements can be grown, but their required chill units will not accumulate every year, which will result in inconsistent cropping.
Cultivars adapted to Florida have been developed by two programs. The Stonefruit Breeding Program operated by the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) has focused on breeding cultivars with chilling requirements ranging from approximately 100 to 450 chill hours. A second cooperative breeding program located at the Attapulgus Research and Education Center involving scientists from UF/IFAS, the University of Georgia (UGA) and the USDA-ARS in Byron, Georgia has focused on breeding peaches with chilling requirements ranging from 350 to 650 chill hours.
A major focus of the UF/IFAS Stonefruit Breeding Program has been the development of non-melting texture cultivars that can be harvested tree ripe. The non-melting texture cultivars ’UFGold’, ‘UF2000’, ‘UFO’, ‘UFBest’, ‘UFSharp’, ‘UFBlaze’, ‘UFBeauty’, ‘UFGlo’, ‘UFSun’, and 'UFOne' have been released. In addition, the non-melting texture nectarine cultivars ‘UFQueen’ and 'UFRoyal', have been released from the UF/IFAS program. For growers in northern Florida and southern Georgia, the cooperative effort has released ‘Gulfprince’, ‘Gulfking’, ‘Gulfcrest’, and ‘Gulfcrimson’ peaches, which require a higher chilling unit accumulation (350–525 chill units). Today, these and other cultivars released by the UF/IFAS Stonefruit Breeding Program are grown in Australia, Spain, Morocco, South Africa, and around the world.
Most peaches grown for fresh fruit consumption are soft when ripe and are said to have melting texture. The UF/IFAS program focuses on breeding of non-melting texture peaches that are firm even when ripe. Non-melting texture peaches are typically clingstone. The term clingstone refers to the texture adhering to the pit making the pit difficult to extract from the flesh when the peach is sliced in half. It appears that the enzymes involved in softening the texture are also involved in facilitating the separation of the flesh from the pit (Bailey and French 1949; Crisosto 2002). Although there is some variation in the degree of flesh adherence (e.g., semi-freestone or semi-clingstone), non-melting texture, freestone peaches are not currently available.
Commercial Peach and Nectarine Cultivars for Northern and North-Central Florida
‘Flordabest’—250 Chill Units
‘Flordabest’ peach was released and patented by UF/IFAS in 2009 and has a fruit development period (FDP) of 82 days from fruit set to harvest. The fruit develop 90%–100% blush, making them very attractive. The fruit are large and have melting, but uniformly firm, yellow flesh, and semi-clingstone pits. The fruit ripen about 7–10 days earlier than the standard peach cultivar ‘TropicBeauty’ in Gainesville, Florida. It is recommended for trial in Gainesville and south to Interstate 4 (Figure 3-1).

Credit: UF/IFAS
‘UFO’—250 Chill Units
‘UFO’ is a non-melting-flesh, peento type peach. Peento peaches have a unique “doughnut” shape. It was released and patented by UF/IFAS in 2002 and produces large, vigorous trees with a semi-upright growth habit. ‘UFO’ produces moderately heavy crop loads of large, firm fruit with a yellow flesh and semi-freestone pits that have an FDP of 95 days. The skin develops 50%–70% blush. This cultivar is particularly susceptible to ethylene, a stress hormone that is released during dormant pruning, and that can result in significant flower bud abortion. Thus, pruning is not recommended except during the summer period (Figure 3-2).

Credit: UF/IFAS
‘Gulfking’—350 Chill Units
‘Gulfking’ is a non-melting-flesh peach cultivar that was released and patented by the joint UF/IFAS, UGA, and USDA-ARS breeding program in 2004 (Krewer et al. 2005). The fruit have exceptional color, with 80%–90% red skin with stripes over a deep yellow ground color. The fruit are also very firm with yellow flesh and are clingstone. The FDP is 77 days, and the fruit develop good size, shape, and color in northern Florida (Figure 3-3).

Credit: UF/IFAS
‘Gulfsnow’—400 Chill Units
‘Gulfsnow’ peach is a 2012 joint release from the UF/IFAS, UGA, and USDA-ARS breeding program. Trees of ‘Gulfsnow’ are vigorous and semi-spreading, producing white, non-melting-flesh fruit. ‘Gulfsnow’ fruit are large, round, and attractive with a 50%–60% blush over a cream background. The fruit are clingstone, with medium-sized, red pits, and have an FDP of 110 days (Figure 3-4).

Credit: UF/IFAS
‘Gulfcrimson’—400 Chill Units
‘Gulfcrimson’ is the third in a series of peach cultivars released and patented by the joint UF/IFAS, UGA, and USDA-ARS Stonefruit Breeding Program, specifically in 2009 (Krewer et al. 2008). ‘Gulfcrimson’ fruit are large for an early-ripening cultivar and have a yellow ground color with 80%–90% red skin. ‘Gulfcrimson’ ripens with the standard peach cultivar ‘JuneGold’ in Attapulgus, Georgia, with an FDP of 95 days and highly consistent cropping, making it a good mid-season replacement for ‘JuneGold’. It also crops reliably in northern Florida (Figure 3-5).

Credit: USDA-ARS
‘UFGlo’—400 Chill Units
‘UFGlo’ is a white-flesh, non-melting peach that was released in 2009. ‘UFGlo’ fruit are large, develop 80%–90% blush over the entire fruit, and are clingstone. The FDP is 80–85 days. ‘UFGlo’ ripens in areas where the standard cultivar ‘Flordaking’ does well, and it complements ‘UFSharp’ peaches in north-central Florida. It produces consistent crops with good yields in northern Florida (Figure 3-6).

Credit: UF/IFAS
‘GulfAtlas’—400 Chill Units
‘GulfAtlas’ peach is a 2014 release from the UF/IFAS, UGA, and USDA ARS joint breeding program. It is a late-season cultivar, with an FDP of 120 days. The fruit have non-melting flesh with clingstone pits and bright yellow flesh. Fruit are very large and round and ripen about 3 weeks after ‘Gulfcrimson’. Ripe fruit have a 75% blush with some red pigmentation in the flesh under the skin. Trees are vigorous and semi-spreading with few blind nodes and good fruit set. ‘GulfAtlas’ is adapted to an area south of Attapulgus, Georgia, to Gainesville, Florida (Figure 3-7).

Credit: USDA-ARS
‘Gulfcrest’—525 Chill Units
‘Gulfcrest’ peach is a 2004 release from the UF/IFAS, UGA and USDA-ARS joint breeding program. It has an FDP of 62–75 days, and the fruit have non-melting flesh with clingstone pits. Ripe ‘Gulfcrest’ fruit have 90%–95% red color over a deep yellow to orange ground color and ripen in early to mid-May in southern Georgia. ‘Gulfcrest’ fruit can be variable in size on the tree. ‘Gulfcrest’ can produce “twiggy” branch growth. ‘Gulfcrest’ is adapted to extreme northern Florida and southern Georgia (Figure 3-8).

Credit: USDA-ARS
‘Sunbest’—225 Chill Units
‘Sunbest’, released in 2001, is a patented nectarine cultivar with yellow, melting flesh and a semi-freestone pit. It develops 90%–100% bright red blush over a yellow ground color and has a FDP of 85–90 days. ‘Sunbest’ fruit ripen in early May in Gainesville, Florida, about 3 days before the standard ‘Sunraycer’ nectarine cultivar. It is superior to and a good replacement for ‘Sunraycer’ nectarine (Figure 3-9).

Credit: UF/IFAS
‘UFRoyal’—250 Chill Units
‘UFRoyal’ is a yellow flesh, non-melting nectarine with an FDP of 85 days. ‘UFRoyal’ fruit are large, with 100% red skin and semi-clingstone pits. Fruit are symmetrically oval, and in Gainesville, Florida, they ripen approximately 1 week before ‘UFQueen’ (below), in early May. ‘UFRoyal’ fruit have excellent firmness and flavor with excellent shipping potential (Figure 3-10).

Credit: UF/IFAS
‘UFQueen’—250 Chill Units
‘UFQueen’ nectarine is a regular bearer of early, large fruit in north-central Florida, with an FDP of 95 days. ‘UFQueen’ trees are semi-upright and are easily pruned to an open vase system. The fruit have non-melting flesh with clingstone pits and yellow flesh color. The fruit are slightly oval with a slight tip and develop 80%–100% red skin over a yellow background. ‘UFQueen’ fruit ripen about 1 week after the standard ‘Sunraycer’ nectarine cultivar in mid-May in Gainesville, Florida (Figure 3-11).

Credit: UF/IFAS
Commercial Peach Cultivars for Central and South-Central Florida
‘UFSun’—100 Chill Units
‘UFSun’ is a non-melting-flesh peach cultivar released in 2004 (Rouse et al. 2004). ‘UFSun’ trees bear heavy annual crops of early-season, medium-sized fruit with yellow flesh and clingstone pits. ‘UFSun’ fruit are uniformly symmetrical and develop 50%–60% red skin with darker red stripes. ‘UFSun’ fruit ripen with those of the standard peach cultivar ‘Flordaprince’ at Immokalee and Gainesville, Florida, with an FDP of 80 days (Figure 3-12).

Credit: UF/IFAS
‘UFBest’—100 Chill Units
‘UFBest’, released by the UF/IFAS breeding program in 2012, is a non-melting-flesh peach cultivar that produces heavy annual crops of large fruit. ‘UFBest’ fruit develop 95%–100% red skin over a yellow ground color, and the flesh is yellow with clingstone pits. ‘UFBest’ fruit ripen 1 week earlier than ‘UFSun’ (mid-April) in Gainesville, Florida, with an FDP of 85 days (Figure 3-13).

Credit: UF/IFAS
‘TropicBeauty’—150 Chill Units
‘TropicBeauty’ is a non-patented peach cultivar released jointly by UF/IFAS and Texas A&M in 1989. The medium-sized, semi-freestone fruit have yellow, melting flesh and develop 70% blush over a yellow ground color. ‘TropicBeauty’ fruit ripen between ‘UFSun’ and ‘UFOne’ and have an FDP of 89 days (Figure 3-14).

Credit: UF/IFAS
‘UFGem’—175 Chill Units
‘UFGem’ was released in 2013 and is a good candidate for the northern part of central Florida. It is a commercial peach cultivar with near 100% blush, average fruit size of 2.5-inch diameter, and symmetrical fruit shape. It is a clingstone peach with non-melting, yellow flesh, and firm texture. The average FDP is 83 days and ‘UFGem’ sets fruit well when minimum nighttime temperatures are above 57 °F (14 °C) (Figure 3-15).

Credit: UF/IFAS
‘UFBeauty’—200 Chill Units
‘UFBeauty’ is a peach cultivar released in 2002 with fruit that have non-melting flesh, clingstone pits, and very symmetrical shape. The flesh of ‘UFBeauty’ fruit is yellow and very firm, and the skin color is near 100% red, with darker red stripes. ‘UFBeauty’ fruit ripen 3 to 4 days after ‘UFGold’ in Gainesville, Florida, with an FDP of 82 days. Cropping of ‘UFBeauty’ has been unreliable in southern Florida when night temperatures during the bloom period are higher than 57 °F (14 °C) (Figure 3-16).

Credit: UF/IFAS
‘UFOne’—250 Chill Units
‘UFOne’ is a non-melting-flesh peach cultivar released by UF/IFAS in 2008. ‘UFOne’ fruit are medium-large, and the trees regularly bear large crops of marketable fruit. ‘UFOne’ fruit are very firm with yellow flesh and semi-clingstone pits and develop 40% red blush over a yellow ground color. ‘UFOne’ fruit have a fairly long FDP of 95 days and ripen with ‘UFBeauty’ (early May) in Gainesville, Florida (Figure 3-17).

Credit: UF/IFAS
Backyard Peach and Nectarine Cultivars for Northern, North-Central, and Central Florida
‘Flordaking’—450 Chill Units
‘Flordaking’ is an older peach cultivar released by UF/IFAS in 1978. It is not patented and is a good selection for backyard production, with large fruit for a mid-season peach cultivar and moderate resistance to bacterial spot. Fruit of ‘Flordaking’ have yellow, melting flesh, and clingstone pits. ‘Flordaking’ fruit develop 70% red blush over a yellow ground color. ‘Flordaking’ has an FDP of 65–70 days and ripens in early May in Gainesville, Florida. One disadvantage of ‘Flordaking’ fruit is high incidence of split pits when crop loads are low (Figure 3-18).

Credit: UF/IFAS
‘Gulfprince’—400 Chill Units
‘Gulfprince’ peach was jointly released by UF/IFAS, UGA, and USDA-ARS in 2002. Trees of ‘Gulfprince’ are large and vigorous with a spreading growth habit. ‘Gulfprince’ fruit are uniform and symmetrical and develop 45%–55% solid red skin. Fruit have non-melting, and yellow flesh with clingstone pits. ‘Gulfprince’ fruit have exhibited some slight browning due to oxidation on soft, ripe fruit. The FDP is 110 days (Figure 3-19).

Credit: USDA-ARS
‘Suncoast’—375 Chill Units
‘Suncoast’ is a non-patented melting-flesh nectarine cultivar released by UF/IFAS in 1995. ‘Suncoast’ trees are vigorous and semi-spreading. Fruit of ‘Suncoast’ have yellow flesh, develop 80%–90% red blush over a yellow ground color, and are semi-clingstone. ‘Suncoast’ fruit are slightly oblong with no sharp tips or bulges and tend to be tart. ‘Suncoast’ leaves and fruit are resistant to bacterial spot. The FDP of ‘Suncoast’ fruit is 77 days, and the fruit ripen in late April to early May in Gainesville, Florida (Figure 3-20).

Credit: UF/IFAS
‘Flordacrest’—350 Chill Units
‘Flordacrest’ is a melting-flesh semi-clingstone peach cultivar released by UF/IFAS in 1988. ‘Flordacrest’ trees are vigorous with a spreading habit. ‘Flordacrest’ fruit have yellow flesh and develop 60%–80% red blush over a bright yellow ground color. It is the best melting-flesh peach currently available for northern Florida. It ripens after ‘Flordaking’ in northern Florida, in early May in Gainesville, Florida, with an FDP of 75 days (Figure 3-21).

Credit: UF/IFAS
‘UFSharp’—325 Chill Units
‘UFSharp’ is a patented, non-melting-flesh clingstone peach cultivar that was released by UF/IFAS in 2006. ‘UFSharp’ trees are vigorous, semi-spreading in nature, and productive. ‘UFSharp’ fruit develop 60% red blush over a deep yellow to orange ground color. ‘UFSharp’ has reliable cropping with excellent fruit size, shape, and firmness, and an FDP of 105 days (Figure 3-22).

Credit: UF/IFAS
‘UF2000’—300 Chill Units
‘UF2000’ is a yellow-flesh, non-melting, clingstone peach, released in 2000. Trees are highly vigorous with a semi-spreading growth habit and produce heavy annual crops of moderately large fruit. ‘UF2000’ fruit are symmetrically shaped and develop 50%–70% solid red skin over a yellow background. ‘UF2000’ fruit ripen mid-season, with harvest occurring from 15–18 days before ‘UFGold’ in mid-late May in Gainesville, Florida (Figure 3-23).

Credit: UF/IFAS
‘UFBlaze’—300 Chill Units
‘UFBlaze’ is a non-melting-flesh, clingstone peach cultivar released in 2002. Trees are highly vigorous with a semi-spreading nature. ‘UFBlaze’ trees produce heavy annual crops of large, early-ripening, attractive fruit with bright red skin over 80%–90% of a bright yellow-orange background and yellow flesh. Fruit are uniform and symmetrical, and they ripen about 7 to 10 days after ‘UFGold’ in early to mid-May in Gainesville, Florida, with an FDP of 83 days (Figure 3-24).

Credit: UF/IFAS
‘Flordadawn’—300 Chill Units
‘Flordadawn’ is a melting-flesh non-patented peach cultivar released by UF/IFAS in 1989. ‘Flordadawn’ trees are vigorous and produce large numbers of flowers with a moderately high fruit set. The bloom period of ‘Flordadawn’ is extended, which can help with fruit set during early spring frosts. The FDP of ‘Flordadawn’ is 60 days, which is the shortest of any named peach cultivar. Fruit of ‘Flordadawn’ develop 80% red blush and have yellow flesh with semi-clingstone pits. However, light crop loads have resulted in as much as 50% split pit incidence. ‘Flordadawn’ can often be found in large stores and nurseries for backyard plantings (Figure 3-25).

Credit: UF/IFAS
‘Sunmist’—300 Chill Units
‘Sunmist’ is a patented melting-flesh nectarine cultivar released by UF/IFAS in 1994. ‘Sunmist’ trees are highly vigorous and have a spreading growth habit. Fruit of ‘Sunmist’ have white flesh, are semi-freestone, and are large for an early-ripening cultivar. Fruit develop nearly 100% red blush and are uniformly symmetrical. ‘Sunmist’ trees and fruit are highly resistant to bacterial spot. The FDP is 85 days, and the fruit ripen in early May in Gainesville, Florida (Figure 3-26).

Credit: UF/IFAS
‘Sunraycer’—275 Chill Units
‘Sunraycer’ is a non-patented melting-flesh nectarine cultivar released by UF/IFAS in 1993. Trees are vigorous and semi-spreading, responding well to open-center pruning systems. ‘Sunraycer’ produces large, semi-clingstone fruit with good firmness and resistance to bacterial spot. ‘Sunraycer’ fruit develop 80%–100% brilliant red blush over a bright yellow ground color and are oval with no sharp tips or suture bulges. ‘Sunraycer’ has an 85-day FDP and ripens in early May in Gainesville, Florida (Figure 3-27).

Credit: UF/IFAS
‘Sunbest’—225 Chill Units
‘Sunbest’ is a patented melting-flesh nectarine cultivar released by UF/IFAS in 2001. ‘Sunbest’ trees are semi-upright and vigorous, responding well to open-center pruning systems. ‘Sunbest’ fruit develop 90%–100% bright red blush over a yellow ground color, are semi-freestone, and resist bacterial spot well. ‘Sunbest’ is intended as a replacement for ‘Sunraycer’ nectarine because of its larger and more attractive fruit. ‘Sunbest’ has an FDP of 85–90 days, and its fruit ripen approximately 3 days before ‘Sunraycer’ nectarine and ‘Flordaglo’ peach in Gainesville, Florida (Figure 3-28).

Credit: UF/IFAS
Backyard Peach Cultivars for Central and South-Central Florida
‘TropicSnow’—225 Chill Units
‘TropicSnow’ was jointly released by UF/IFAS and Texas A&M in 1989. Its fruit have white, melting-flesh, and semi-freestone pits. ‘TropicSnow’ fruit develop 40%–50% red blush over a creamy white background and have very low acid combined with excellent sweetness. ‘TropicSnow’ has an FDP of 90–97 days in Gainesville, Florida (Figure 3-29).

Credit: UF/IFAS
‘UFGold’—200 Chill Units
‘UFGold’ is a non-melting, yellow-flesh clingstone peach released by UF/IFAS in 1996. ‘UFGold’ trees bear heavy annual crops of large fruit. Fruit are symmetrical and develop 70%–90% blush over an orange-yellow ground color. ‘UFGold’ fruit ripen approximately 80 days after bloom, in early May in Gainesville, Florida (Figure 3-30).

Credit: UF/IFAS
‘Flordaprince’—150 Chill Units
‘Flordaprince’ was released by UF/IFAS in 1982, and its fruit have melting flesh. It has been a standard low-chill peach cultivar worldwide and is one of the earliest-ripening peach cultivars. The fruit develop 80% red blush with dark red stripes over a yellow ground color. ‘Flordaprince’ fruit are large, uniformly firm, and yellow, with semi-clingstone pits. The fruit ripen about 7–10 days earlier than ‘TropicBeauty’ in Gainesville, Florida, with an FDP of 78 days (Figure 3-31).

Credit: UF/IFAS
‘Flordaglo’—150 Chill Units
‘Flordaglo’ is a melting-flesh peach cultivar released by UF/IFAS in 1988. The fruit develop 50%–60% red blush with stripes over a white ground color. ‘Flordaglo’ fruit are early-ripening and semi-clingstone. They are resistant to bacterial spot. ‘Flordaglo’ fruit ripen in early May in Gainesville, Florida, approximately 78 days after full bloom. Due to its melting-flesh fruit texture and the tendency of the fruit to show bruises and abrasions easily, ‘Flordaglo’ fruit are a poor choice for commercial production but are ideal for backyard or u-pick operations (Figure 3-32).

Credit: UF/IFAS
Plum Cultivars Adapted to Northern, North Central, and Northwestern Florida
‘Gulfbeauty’
‘Gulfbeauty’ plum was released in 1998 and patented by UF/IFAS. Its chilling requirement is about 225 hours. Fruit color is dark reddish purple, and the flesh is yellow with a green hue (Figure 3-33). The skin is sour, which is common in Japanese plums, but the flesh is sweet, sub-acid, and firm when ripe. The fruit are clingstone, and the flesh clings to the stone even when soft ripe. Fruit are round and medium-sized (1¾ inch in diameter) and weigh from 55 to 70 grams. Bloom and cross-pollination occur with all other ‘Gulf’ series plums. Fruit set is good, with flowers formed on spurs and the previous season’s shoots. ‘Gulfbeauty’ is the earliest plum to ripen from the UF/IFAS breeding program, with a fruit developmental period of 75 days in Gainesville, Florida.
Ripening occurs about 5 days before ‘Gulfruby’ and about 8–12 days before ‘Gulfblaze.’ As the fruit approach full ripe, their color becomes noticeably darker. Ripe fruit will hang on the tree for 7–10 days. Quality is good, especially for an early-ripening plum. During its season, no other fresh plums are available. Trees are vigorous with tall upright shoots and are semi-spreading. In the absence of freezing conditions, thinning is required to obtain adequate size and prevent limb breakage. Trees are very resistant to bacterial canker and moderately resistant to plum leaf scald.

Credit: Paul Miller, UF/IFAS
‘Gulfblaze’
‘Gulfblaze’ plum was released and patented by UF/IFAS in 1997 (Figure 3-34). Fruit ripen in the middle of the Florida plum season. The chilling requirement for ‘Gulfblaze’ is about 250 hours. Bloom and cross-pollination occur with all other ‘Gulf’ series plums. Fruit set is good with flowers formed on spurs and the previous season’s shoots. Fruit are very firm, average in size (1 7/8 to 2 inches in diameter), and weigh 70 to 80 grams. Fruit are round and semi-freestone with flesh weakly attached to the pit when ripe. Fruit color is dark red to purple, and the flesh is orange, sweet, and sub-acid (Figure 34). The skin is sour, although overall fruit quality is good. Fruit ripen 8 to 14 days after ‘Gulfbeauty’ in Gainesville, Florida, with a fruit developmental period of 95 days. Bloom, pollination, fruit set, and ripening characteristics are the same as for ‘Gulfbeauty’. The leaves, stems, and fruit have similar resistance to bacterial canker and plum leaf scald as ‘Gulfbeauty’.

Credit: Mercy A. Olmstead, UF/IFAS
‘Gulfrose’
‘Gulfrose’ was released and patented by UF/IFAS in 2002 and ripens about 1 week later than ‘Gulfblaze’, with a fruit developmental period of 95 days in Gainesville, Florida (Figure 3-35). The chilling requirement is about 275 hours. The fruit are nearly round, semi-freestone, average in size (1 7/8 to 2 inches in diameter), and weigh 70 to 80 grams. The skin is dark reddish purple, and the flesh is blood red in color (Figure 35). Fruit quality is high with good firmness and shelf life. The fruit have a sweet, aromatic flesh and a moderately sour skin. There is none of the bitter aftertaste common in other blood plums such as ‘Mariposa’. Bloom, pollination, fruit set, and ripening qualities are the same as for those of ‘Gulfbeauty’. Trees are moderately vigorous, semi-spreading, and precocious, bearing the second year after planting. ‘Gulfrose’ has similar resistance to bacterial canker as ‘Gulfbeauty’; however, it is less tolerant to plum leaf scald than ‘Gulfblaze’. ‘Gulfrose’ susceptibility to leaf scald is similar to that of ‘Gulfgold’, limiting tree longevity.

Credit: Mercy A. Olmstead, UF/IFAS
‘Gulfruby’
‘Gulfruby’ originated at the UF/IFAS breeding program but was not released by the university because of its susceptibility to bacterial canker. It was first propagated along with ‘Gulfgold’ in 1982 by Grand Island Nursery in Umatilla, Florida. It is not patented and is, therefore, a public cultivar. Bacterial canker readily occurs on leaves and small twigs of ‘Gulfruby’ and is aggravated by frequent summer rains. Generally, the tree may survive from 5 to 8 years and provide several crops of early-ripening plums. Fruit are round, medium-sized, and up to 2 inches in diameter (Figure 3-36). The flesh is sweet, yellow with a greenish tinge, and adheres to the small pit when fruit are soft ripe. Skin color is red to purple with sour characteristics. Fruit will hang on the tree 3–5 days after full red skin color develops. ‘Gulfbeauty’ fruit will ripen 7 to 10 days before ‘Gulfblaze’ in Gainesville, Florida. The tree is not as vigorous as ‘Gulfbeauty’, nor does it possess as much of the strong upright shooting tendencies of ‘Gulfbeauty’. ‘Gulfruby’ has more problems with bacterial canker than the other low-chill cultivars recommended for trial. Bacterial canker can cause cankers on the wood and significant leaf damage leading to defoliation, which can result in sunburn on the fruit due to leaf loss.

Credit: Paul Miller, UF/IFAS
‘Gulfgold’
‘Gulfgold’ is a yellow skin plum, but the fruit develop a red blush as ripening progresses. Fruit flesh is yellow and semi-soft when ripe. Although it was developed by UF/IFAS, it was not patented. Fruit ripen in late May to mid-June in Gainesville, Florida. They are the sweetest of the ‘Gulf’ series. ‘Gulfgold’ is susceptible to plum leaf scald, which generally limits tree life in Florida. Trees are dwarf in growth habit. Bloom is after ‘Gulfruby’ by a few days, and ‘Gulfgold’ will cross-pollinate with the other ‘Gulf’ plums.

Credit: Daleys Fruit. https://www.daleysfruit.com.au/buy/plum-gulfgold-tree.htm
Plum Cultivars Conditionally Recommended for Trial for Northern Florida Due to a Higher Chilling Requirement
Plum cultivars with the Au prefix have been released from the University of Auburn plum breeding program, and ‘Robusto’, ‘Segundo’, and ‘Byrongold’ are from the USDA-ARS Stonefruit Breeding Program in Byron, Georgia. They can cross-pollinate each other. Florida nurseries may sell other cultivars such as ‘Scarlet Beauty’; however, there is no data available on their tree or fruit production characteristics.
‘Au-Homeside’
‘Au-Homeside’ produces a light red plum with amber flesh. Fruit are oval and about 2 1/3 inches in diameter. Fruit are attractive and overall quality is good. Fruit ripen in mid-June and tend to size well before color is achieved. Trees are not vigorous, but ‘Au-Homeside’ is tolerant to plum leaf scald.
‘Au-Producer’
‘Au-Producer’ fruit have dark red skin, and the flesh is also red. Fruit are small, round, and less than 2 inches in diameter. Fruit quality is high, and the fruit ripen in mid-June. Tree vigor is moderate, but trees require heavy thinning. ‘Au-Producer’ is tolerant to plum leaf scald. Chilling requirement is about 750 units.
‘Au-Roadside’
‘Au-Roadside’ produces a magenta fruit with a red flesh color. Fruit are oval and less than 2 inches in diameter. Fruit quality is very good, and fruit ripen in mid-June. Trees are highly vigorous. Fruit tend to be too soft for commercial shipping. ‘Au-Roadside’ is tolerant to plum leaf scald. Chilling requirement is about 750 units.
‘Au-Rosa’
‘Au-Rosa’ produces red fruit with some light-yellow areas. Fruit are round and 2 inches in diameter. Fruit quality is good, and fruit are attractive. Fruit ripen in mid-June. ‘Au-Rosa’ is resistant to plum leaf scald. Chilling requirement is about 750 units.
‘Au-Rubrum’
‘Au-Rubrum’ skin color is maroon and flesh color is red. Fruit are round and 2 inches in diameter. Ripening date is mid-June. Tree vigor is good. ‘Au-Rubrum’ is tolerant to plum leaf scald. Chilling requirement is about 750 units.
‘Byrongold’
‘Byrongold’ produces fruit with yellow skin and flesh. Fruit develop red blush during the latter part of ripening. Fruit are round and approximately 2 inches in diameter. Fruit ripen in late June to early July with good firmness and fruit quality. Trees are highly vigorous but may have some problems with leaf scald. Chilling requirement is about 450 units.
‘Excelsior’
‘Excelsior’ is a native plum discovered by George Tabor of Glen St. Mary’s Nursery and requires approximately 400 chilling units. Both the flesh and fruit skin are yellow. The fruit size is about 2 inches. The flesh is somewhat translucent and watery.
‘Methley’
‘Methley’ is an older cultivar that is no longer recommended because of small fruit size, lack of firmness, and susceptibility to plum leaf scald and other diseases of bacterial origin. It is self-pollinating. Chilling requirement is about 650 units.
‘Robusto’
‘Robusto’ produces a red fruit with yellow flesh and ripens in early June. The tree blooms in early March and requires approximately 400 to 500 chill units.
‘Santa Rosa’
‘Santa Rosa’ produces a purple/red colored fruit with red flesh. Santa Rosa is an older cultivar that is no longer recommended because of susceptibility to plum leaf scald and other diseases of bacterial origin. Chilling requirement is about 650 units.
‘Segundo’
‘Segundo’ is a red plum with yellow flesh that ripens in mid- to late June. The fruit are somewhat soft, and the tree requires approximately 400 to 500 chilling units.
Additional Reading
Bailey, J. S., and A. P. French. 1933. The Inheritance of Certain Characteristics in the Peach.” Proceedings of the American Society for Horticultural Science 29:127–130.
Crisosto, C. H. 2002. “How do we increase peach consumption?” Acta Horticulturae 592:601–605. https://doi.org/10.17660/ActaHortic.2002.592.82
Krewer, G., T. Beckman, J. Chaparro, and W. Sherman, 2005. “‘Gulfking’ and ‘Gulfcrest’, New Peaches for the Lower Coastal Plain.” HortScience 40:882. https://doi.org/10.21273/HORTSCI.40.3.882d
Krewer, G. W., T. G. Beckman, J. X. Chaparro, and W. B. Sherman. 2008. “‘Gulfcrimson’ Peach.” HortScience 43:1596–1597. https://doi.org/10.21273/HORTSCI.43.5.1596
Rouse, R. E., W. B. Sherman, and P. M. Lyrene, 2004. “‘UF-Sun’ Peach.” Journal of the American Pomological Society 58:108–110.
4. Postharvest Physiology of Stonefruits
Mark A. Ritenour and Jeffrey K. Brecht
Introduction
Stonefruits are relatively soft-fleshed and highly perishable (Figure 4-1). Other chapters in this handbook cover some aspects of stonefruit physiology including fruit maturity, quality attributes, and physiological disorders. This chapter will cover physiological aspects of fruit development and postharvest changes related to ripening and softening.

Credit: Mark A. Ritenour, UF/IFAS
Fruit Development
Peach trees bloom early, before there is extensive leaf development, and so initial fruit set is fed by starch reserves stored in the woody tissues from the previous season’s growth. Thus, tree health and carbohydrate reserves from last season’s growth affect the current season’s crop. Fruit development is ultimately fueled by sunlight harvested by the leaves through photosynthesis during the growing season. The ability of fruit to continue development and successfully compete for the limited resources produced by the leaves depends largely on the proximity of fruit to the supplying leaves (leaves preferentially supply fruit closest to them) and the number of fruit vying for the limited resources. When resources are insufficient, fruit may drop (abscise) prematurely or may not obtain desirable sizes. This is why thinning fruit results in larger fruit size.
After bloom and successful fruit set, fruit growth goes through three stages:
- Rapid fruit growth from cell division and elongation; the duration of this stage is uniform among cultivars.
- Fruit dry weight increases without much increase in fruit size while imported carbohydrates fuel pit (stony endocarp) development and hardening; the duration of this phase is what determines early-, middle-, and late-season cultivars.
- Fruit enlargement and increase in sugar content in the mesocarp (flesh) before harvest; the fruit can begin to ripen during this stage.
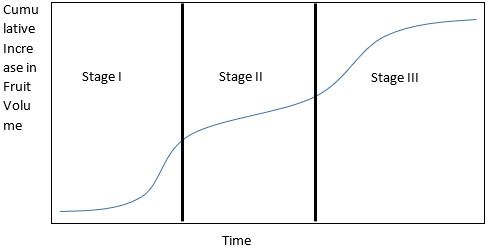
Credit: UF/IFAS
It is important to avoid resource (carbohydrate) limitations throughout the growth period because a lack of carbohydrate at any point during growth can prevent fruit from reaching a desirable size. However, because maximum fruit size is related to the number of cells within the fruit and the first stage of development is the one when cell division occurs, resource limitations during stage I (and especially later in this stage) are of primary concern. Resource limitations during stage I most permanently reduce maximum fruit size. Therefore, fruit thinning, if practiced, should occur before the second half of stage I to reduce resource competition, allowing remaining fruit an adequate supply of resources for maximum cell division. Sugar accumulation within the fruit is most rapid later in fruit development, reaching a maximum just before the onset of the ethylene climacteric (explained below) and ripening. Thus, delaying harvest results in sweeter fruit, but at the risk of the fruit softening to the point that they are easily injured during postharvest handling and marketing.
Fruit Ripening
Most stonefruit except cherries and a few plum cultivars are classified as climacteric fruit (Table 4-1) because their respiration rates increase to a peak (the climacteric) during ripening. This increase in respiration is accompanied by an increase in a gaseous plant hormone called ethylene. The magnitude of the respiratory climacteric peak varies depending on the cultivar, but stonefruits are generally considered to have moderate (10–20 mg CO2/kg-hr) respiration rates. Ethylene production among the climacteric stonefruit species is considered high (10–100 µL/kg-hr) when going through their climacteric. Ethylene actually initiates and coordinates the ripening process in these fruits (e.g., softening and characteristic color and aroma development). Once the ripening is underway, this type of fruit can continue to ripen normally whether it remains attached to the plant or not. Other climacteric fruits include apples, avocados, bananas, mangoes, and pears.
The other type of fruit, called “nonclimacteric,” has a declining pattern of respiration during fruit ripening. These fruits produce very little ethylene, with no change in its production rate during ripening. For example, the ethylene production rate of the nonclimacteric cherry is very low: <0.1 µL/kg-hr. Nonclimacteric fruits are not able to ripen normally unless they remain attached to the plant, so they must always be harvested when fully ripe; other examples include citrus, grapes, and strawberries. While ethylene stimulates and coordinates the ripening of climacteric fruits, it plays no role in nonclimacteric fruit ripening. Figure 4-3 illustrates the different patterns of respiration and ethylene production during ripening for climacteric and nonclimacteric fruits.
Exposing unripe climacteric fruits to ethylene in order to stimulate the onset of ripening is a common commercial practice that is used for some climacteric fruits like bananas and tomatoes. However, recent Florida peach cultivars developed by the University of Florida have the “non-melting-flesh” trait, which allows their fruit to be harvested when they are close to fully ripe because they remain quite firm well into the ripening process. Conversion of starch to sugar is an important process during ripening of some climacteric fruits (e.g., apple and banana), but stonefruit generally contain very little starch before ripening; instead, stonefruits continue to accumulate sugar from photosynthesis while ripening on the tree. Thus, harvesting Florida peaches when they are unripe and using ethylene treatment to promote ripening is not necessary and is not a recommended practice. Leaving fruit on trees to ripen is possible now that non-melting-flesh Florida peaches are available. Peaches left to ripen on the tree are of greatly improved quality compared to peaches grown in most other places.
Table 4-1. Examples of different types of stonefruits and their classification as either climacteric or non-climacteric.
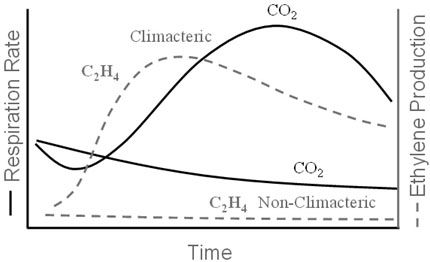
Credit: UF/IFAS
Once climacteric fruits are physiologically mature, or when they have achieved nearly their full size, the ripening process is the same whether the fruit remain on the tree or are harvested, including the changes in color, taste, aroma, and flesh texture. This means that climacteric fruits can be harvested when they are mature but unripe, and then the remainder of the ripening can be controlled (slowed) by using rapid cooling and handling at low temperatures to extend shelf life with acceptable quality. However, since peaches continue to accumulate sugar on the tree during ripening, early harvesting results in fruit that are less sweet than tree-ripe fruit. Non-climacteric fruits can only complete ripening on the tree, and non-climacteric fruits harvested prior to full ripeness will never advance beyond their at-harvest ripeness stage, ensuring poor quality.
Stonefruit shelf life is generally between 1 to 5 weeks depending on the type of stonefruit and the cultivar. Climacteric stonefruits that are harvested immature will not ripen to acceptable quality, while those harvested after ripening begins in the orchard may soften too much and become too easily injured during postharvest handling, transportation, and marketing.
Ripening of climacteric fruits harvested mature but unripe can be hastened by postharvest exposure to ethylene if desired, but the natural pace of ripening is usually sufficient, and accelerated ripening often leads to problems during distribution. The optimum temperature range for ripening stonefruits is 68 °F to 72 °F (20 °C to 22 °C) with relative humidity of 90%–95%. Temperatures above 77 °F (25 °C) reduce the rate of ripening, induce off flavors, and promote irregular ripening, while temperatures above 86 °F (30 °C) inhibit ethylene biosynthesis and thus, inhibit ripening. While inhibitors of ethylene action (e.g., methylcyclopropene [MCP]) have been used effectively to delay ripening and extend shelf life in other climacteric commodities such as apple, they are less effective for use with stonefruit. Using ventilation or scrubbing to remove ethylene from the postharvest environment can also slow the ripening of climacteric fruits.
Depending on the cultivar, the use of modified or controlled atmosphere (MA or CA) conditions containing 1%–5% O2 plus up to 5% CO2, or 3%–10% O2 plus 10%–15% CO2 for cherry, can supplement temperature management by further slowing respiration and ethylene production, delaying softening; possibly inhibiting the development of internal breakdown (chilling injury); and extending overall shelf life. However, the benefits of MA and CA are often not substantial, and such atmospheres also can cause fruit to develop off flavors. Thus, there is very limited use of CA or MA for stonefruit. It is used for occasional marine transport and sometimes in combination with pallet wraps for cherries. The most successful strategy to extend the market window of stonefruit has been the development of cultivars that mature at different times so that there is a continuous supply of product coming to maturity over longer periods of the season. This has proven much more successful than developing techniques to store the fruit for extended periods.
Fruit Softening: Melting-Flesh Peaches vs. Non-Melting-Flesh Peaches
Melting-flesh peaches – This is the traditional type of peach. As melting-flesh peaches begin to ripen, there is an initial “softening stage” during which flesh firmness initially decreases slowly; however, this softening eventually becomes rapid (the “melting stage”). Stonefruit softening is related to the work of a number of different cell-wall enzymes that break down pectic and other carbohydrate polymers in the cell walls. The cell walls almost disappear, basically leaving only sacs of juice held together by the fragile remnants of the cell walls.
Non-melting-flesh peaches – Non-melting flesh in peaches is a natural mutation in which one of the most important enzymes involved in the melting stage of fruit softening (endopolygalacturonase) is absent. The first softening stage during which flesh firmness initially decreases slowly is the same as for melting-flesh fruit, but with the final melting stage being largely absent, non-melting peaches retain some firmness when they are fully ripe. The non-melting trait has traditionally been exploited for canning peach cultivars, but the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) Stonefruit Breeding Program has used it to develop fresh-market cultivars that are more suited for tree-ripe marketing because the ripe fruit remain firm enough for marketing.
Stony hard (hd) peaches – Stonefruit cultivars with this softening trait are characterized by the absence of both ethylene production at ripening and postharvest softening (Ramina et al. 2008). Stony hard is a “slow-ripening” mutant, which means that eventually ethylene will rise, but only to 15%–33% of normal rates. The ripening process in stony hard fruit is slowed sufficiently so that producers are able to harvest partially ripe fruit with a long remaining shelf life. Exposure to ethylene gives a non-climacteric physiological response, meaning that the fruit do not respond by ripening faster.
Additional Reading
Bassi, D., and R. Monet. 2008. “Botany and Taxonomy.” In The Peach: Botany, Production and Uses, edited by Desmond Layne and Daniele Bassi, 1–36. Wallingford, Oxfordshire, GBR: CABI Publishing. https://doi.org/10.1079/9781845933869.0001
Ramina, A., P. Tonutti, and B. McGlasson. 2008. “Ripening, Nutrition and Postharvest Physiology.” In The Peach: Botany, Production and Uses, edited by Desmond Layne and Daniele Bassi, 550–574. Wallingford, Oxfordshire, GBR: CABI Publishing. https://doi.org/10.1079/9781845933869.0550
5. Stonefruit Harvest Operations
Jeffrey K. Brecht and Mark A. Ritenour
When to harvest is one of the most important decisions a grower faces when it comes to providing the marketplace with superior-quality fruit. Optimum harvest maturity corresponds to maximum taste and storage quality (i.e., adequate shelf life). Harvest maturity determines a fruit’s postharvest potential. Stonefruit that are harvested too early have poor flavor potential and greater susceptibility to physiological disorders, abrasion injury, and water loss. With harvest of immature stonefruit, the ability of those fruit to ripen properly can be compromised. Stonefruit that are picked before their optimum maturity may eventually ripen but will develop inferior flavor and aroma, show increased susceptibility to chilling injury caused by low temperatures during transport, and have shortened shelf life.
On the other hand, delaying harvest too long so that ripening is fairly advanced results in greater susceptibility to bruising and decay and possible off-flavor development in overripe fruit. The ability to harvest fruit tree ripe is a major advantage of non-melting-flesh stonefruit cultivars such as those recently released by the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) Stonefruit Breeding Program over traditional melting-flesh types. Non-melting-flesh stonefruit cultivars are typically much firmer and less susceptible to bruising than melting-flesh cultivars at comparable ripeness stages. Melting-flesh cultivars need to be harvested before ripening gets substantially underway because excessive softening limits their shelf life, while non-melting-flesh cultivars can be harvested at a riper stage and still be firm enough to withstand handling.
Recommended Harvest Practices for Yielding High-Quality Stonefruit
Worker Training: Harvest and Sanitation Practices
Because of the seasonal nature of the stonefruit harvest, most farms employ temporary labor. In many cases, temporary harvest personnel return year after year to work in the farms. However, the seasonal nature of the harvest requires a special focus on yearly retraining of harvest crews to assure optimum-quality stonefruit (Figure 5-1). Training must include harvest maturity indicators, defect elimination, good sanitation practices, and worker safety.

Credit: Mark A. Ritenour, UF/IFAS
Fruit Selection, Including Maturity Stage
The stage of maturity of stonefruit at the time of harvest is crucial for the eating quality of the ripe fruit. Selection of the appropriate fruit maturity can be based on several parameters, including fruit size (Figure 5-2) and shape, fruit location on the tree (top and outside fruit normally mature first), external ground color (Figure 5-3), firmness (Figure 5-4), flesh color development (Figure 5-5), soluble solids content (SSC), titratable acidity (TA), and SSC:TA ratio. Although the parameters employed for each cultivar of peach, nectarine, or plum grown commercially might vary somewhat, all commercial growers use one or more of these parameters as an aid to harvest.

Credit: Mark A. Ritenour, UF/IFAS

Credit: Sherry Kao, UF/IFAS
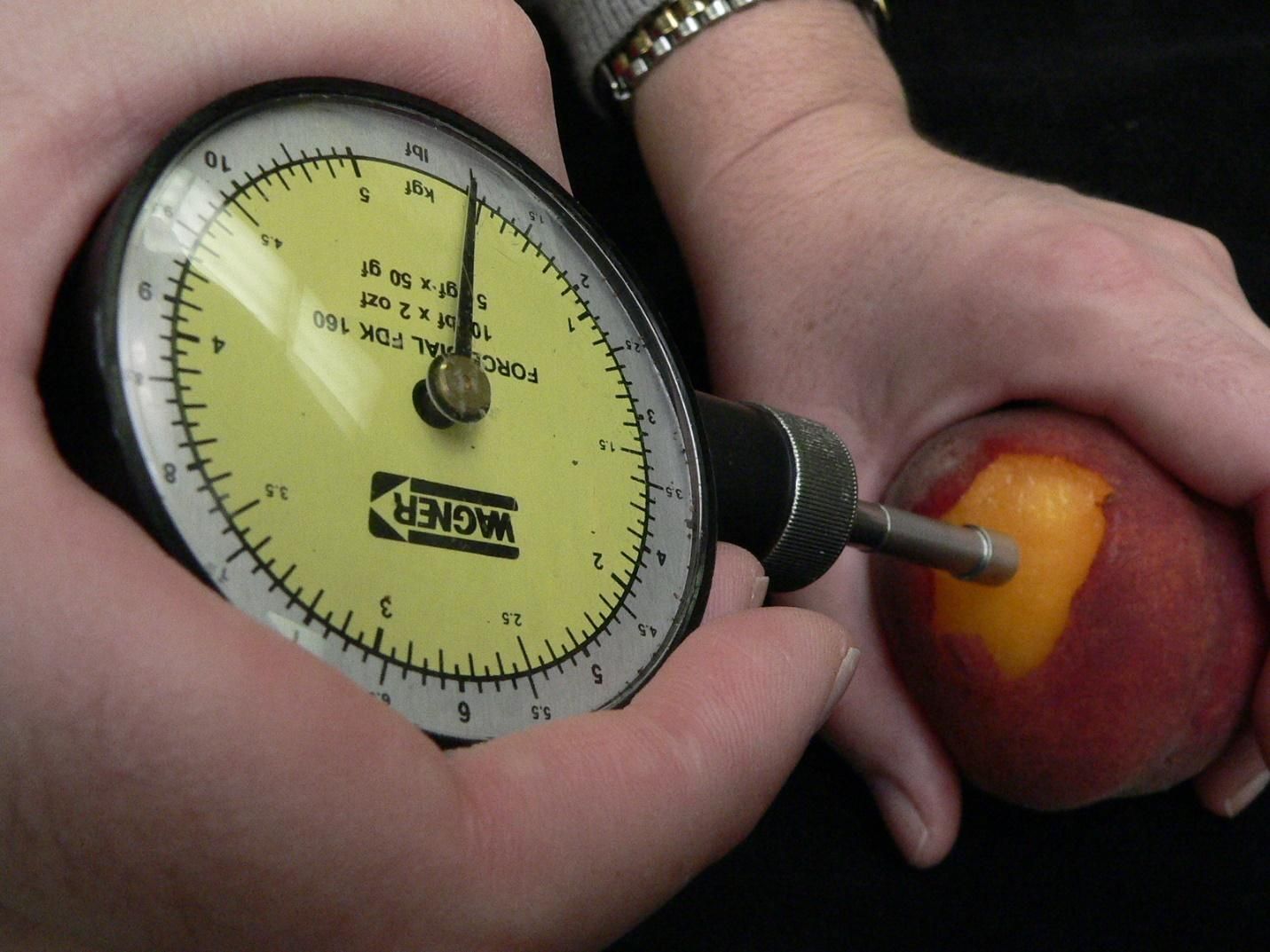
Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Sherry Kao, UF/IFAS
The most widely used harvest maturity index for stonefruits has traditionally been the ground color “break” from green to yellow (Figure 5-3). The ground color, or background color, is the color of the exterior portion of the fruit that is not obscured by the red “blush.” The ground color is a nondestructive indicator and tends to be the indicator pickers most easily understand. The blush that develops on most stonefruits is related to light exposure and is not an indicator of fruit maturity or ripeness. The best ground color at harvest varies by cultivar, growing area, and intended market, so workers should be shown examples before harvest begins.
Many, if not most, newer stonefruit cultivars have complete or nearly complete blush coverage, which makes the use of the ground color as a harvest maturity index problematic. For those cultivars, flesh firmness (Figure 5-4) is the next best maturity index. Research at UF/IFAS has shown that the firmness at either the cheek or blossom end of the fruit is most consistently highly correlated with peach maturity. The ground color is not consistently well-correlated with maturity, probably because it is not consistently apparent.
Since stonefruit softening begins at the blossom end of the fruit, it is fairly easy for a worker to judge fruit firmness at the blossom end with the thumb of their picking hand before removing the fruit from the tree (Figure 5-6). Just as for ground color, workers must be trained to subjectively recognize the range of firmness desired for each cultivar and intended market. The susceptibility of the fruit to bruising determines the minimum firmness at harvest, which in turn determines whether the fruit can be successfully transported to the packinghouse and run over the packingline without incurring injury. Bruising thresholds vary substantially among stonefruit cultivars and therefore must be determined for each cultivar and operation.

Credit: Mark A. Ritenour, UF/IFAS
In addition to cultivar differences, growing regions, climatic conditions, and agronomic practices also influence the expression of maturity indicators in stonefruits. Therefore, growers must validate which parameters prove most effective and dependable for their own conditions.
For details on how to subjectively and objectively determine fruit maturity and ripeness, including how to measure SSC, TA, color, and fruit firmness, see Chapter 2: Stonefruit Quality – Evaluation and Measurement.
Picking and Fruit Accumulation Procedures
Once the decision to harvest stonefruit has been made based on maturity index interpretation, harvest crews should follow recommended picking and field accumulation procedures. The act of picking a peach involves several actions that include judging the ground color development (if not obscured by the red blush coloration), determining that the fruit is within the desired size range and is not defective in any way, grasping the fruit and squeezing slightly to judge the firmness, and finally, twisting and snapping the fruit to remove it from the branch. A mature peach should detach fairly easily from the tree.
In commercial operations, the use of harvest aids such as ladders, harvest bags, buckets, and totes is very common and helps speed up harvest (Figures 5-7 and 5-8). The size of the harvest container and allowable depth of accumulated fruit within the container will depend primarily on the sensitivity of the fruit to bruising. However, one may also want to limit the size of the container because harvesters tend to swing and bang large containers of fruit against various objects (e.g., their legs, ladder rungs, and the sides of bins), leading to fruit damage.
Dirty surfaces tend to be more abrasive than clean surfaces. Therefore, keeping all harvest equipment clean helps reduce abrasion damage to the fruit. To help prevent harvesters from possibly contaminating their hands (and subsequently fruit) with soil-borne pathogens carried on shoes, instruct harvesters not to carry or handle ladders by the rungs. Supervision of the harvest crew is critical to prevent fruit injury and to avoid potential risks related to food safety.

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mike Burton, UF/IFAS

Credit: Mike Burton, UF/IFAS
Peaches are hand-picked using bags, baskets, or totes. Most commercial peach operations use picking bags and bins for harvest (Figures 5-8 and 5-9). Peaches should be poured carefully from the bags or baskets into bins that are on the top of trailers between rows in the orchard. The fruit should not be allowed to drop or roll free during the transfer from bags or baskets to bins. If totes are being used, they are placed directly inside the bins or on carts (Figure 5-10). Baskets are placed on top of modified trailers. Fruit picked at advanced maturity stages and white-fleshed peaches are generally picked and placed into baskets or totes.

Credit: Jeffrey K. Brecht, UF/IFAS
Wood field bins tend to cause abrasion injury to peaches, especially if rough roads and trucks with standard suspension allow the fruit to vibrate excessively. The corners of ventilation slots on the bin sides and bottoms should be rounded off using a router to remove sharp edges that may cut the fruit. Plastic liners on the sides of field bins reduce vibration injury; 5/16-inch-thick plastic bubble liners are even more effective in reducing fruit injury than plastic sheeting. Bottom liners have not been found to reduce vibration injury. Bin liners must have slits that match up with the ventilation slots. Farm roads should be graded to smooth the ride, and rough roads elsewhere should be avoided. Trucks should be equipped with air ride suspension systems. Tire pressures may also be reduced for a more gentle ride. Vehicle speed limits relative to road conditions should be established and strictly enforced.
Plastic field bins are generally more expensive than wooden ones, but the advantages of plastic are many. Plastic bins are lighter and less abrasive to the fruit. They have lower maintenance costs and typically have greater ventilation area, which helps with temperature management. Plastic bins also do not absorb moisture from the fruit and are easier to clean and sanitize than wooden bins. Some plastic field bins are collapsible, which means they take up less space during transport and storage between uses (Figure 5-11).

Credit: Mark A. Ritenour, UF/IFAS
On most commercial farms, fruit may wait from 30 minutes to up to 6 hours before they are transported to the packinghouse. Time in the orchard before transporting harvested stonefruit to the packinghouse should be minimized, and the fruit should be accumulated in the shade (Figure 5-10) to protect them from exposure to direct sunlight while they await transport to the packinghouse. High field temperatures result in accelerated metabolism, which shortens fruit shelf life.
Transport to Packinghouses
Under ideal conditions, the trees being harvested should be located a short distance from the packinghouse. If fruit transport requires traveling lengthy distances, producers should observe the considerations below to minimize the adverse effects on quality that transport to the packinghouse might cause to the stonefruit.
Protect Fruit from Direct Sunlight
After harvest, high air temperatures and direct exposure to sunlight increase stonefruit respiration and water loss, resulting in loss of shelf life. Stonefruit that are directly exposed to the sun can also develop “sun burn,” in which surface tissue is killed by high temperatures. Transport vehicles should be covered to protect the top layers of fruit from direct exposure to sunlight while they are in transit to the packinghouse.
Select a Transport Method That Allows Ventilation
In addition to protection from sunlight, it is important to select a transport truck that allows air circulation while the fruit are in transit, especially while waiting for unloading at the packinghouse. Waiting times for unloading fruit in a typical packinghouse can vary greatly, depending on the volume of fruit being harvested at any time, so protecting the fruit from overheating is critical.
When Possible, Harvest and Transport Stonefruit During Cooler Hours of the Day
It is advisable to harvest stonefruit in the morning and transport them to the packinghouse as quickly as possible. Transport during cooler hours of the day favors lower fruit temperatures that could better preserve the quality and shelf life of stonefruit.
Schedule Deliveries to the Packinghouse
Most commercial packinghouses use some sort of harvest schedule that allows for controlled quantities of fruit to arrive at the reception line. Whenever volumes of fruit harvested exceed the capacity of reception lines, the result is a longer-than-normal wait before fruit are unloaded. During their wait inside transport trucks, stonefruit may be exposed to high ambient temperatures and poor ventilation.
A possible practice to consider is for reception personnel to work night shifts to favor cooler ambient temperatures while stonefruit wait for unloading. Night shifts likely mean higher labor costs for the reception line; however, the benefits to stonefruit quality and shelf life resulting in reduced losses and greater sales will most likely outweigh those labor costs.
Additional Reading
Kader, A. A., and F. G. Mitchell. 1989. “Managing the Crop during and after Harvest.” In: J.H. LaRue and R.S. Johnson 157–229. Peaches, Plums, and Nectarines. Growing and Handling for Fresh Market. Publ. 3331. Univ. Calif., Agric. Natural Resources, Oakland, Calif.
Thompson, J.F. 2002. Harvesting systems, p. 63–65. In: A.A. Kader (ed.). Postharvest Technology of Horticultural Crops. Publ. 3311. Univ. Calif., Agric. Natural Resources, Oakland, Calif.
6. Stonefruit Packing Operations
Mark A. Ritenour and Jeffrey K. Brecht
Standard stonefruit packing operations usually include sorting the fruit by size (sizing), removing unmarketable fruit (grading), and packing the fruit for shipment. Depending on fruit characteristics and the packing method, other postharvest treatments such as washing, waxing, and fungicide treatments may also be applied. The choice to use any of the above operations must be made based on whether they add value to the product so that buyers are willing to pay extra for the services. Postharvest operations that do not add value to the product should not be undertaken. With this in mind, a wide variety of systems have been developed to pack stonefruit depending on fruit condition (e.g., maturity and firmness), peak throughput of fruit to be handled, and possible postharvest treatments (e.g., washing and waxing) desired by the marketplace. The two most common stonefruit packing systems are the “mechanized packing” and “ranch packing” systems.
Mechanized Packing
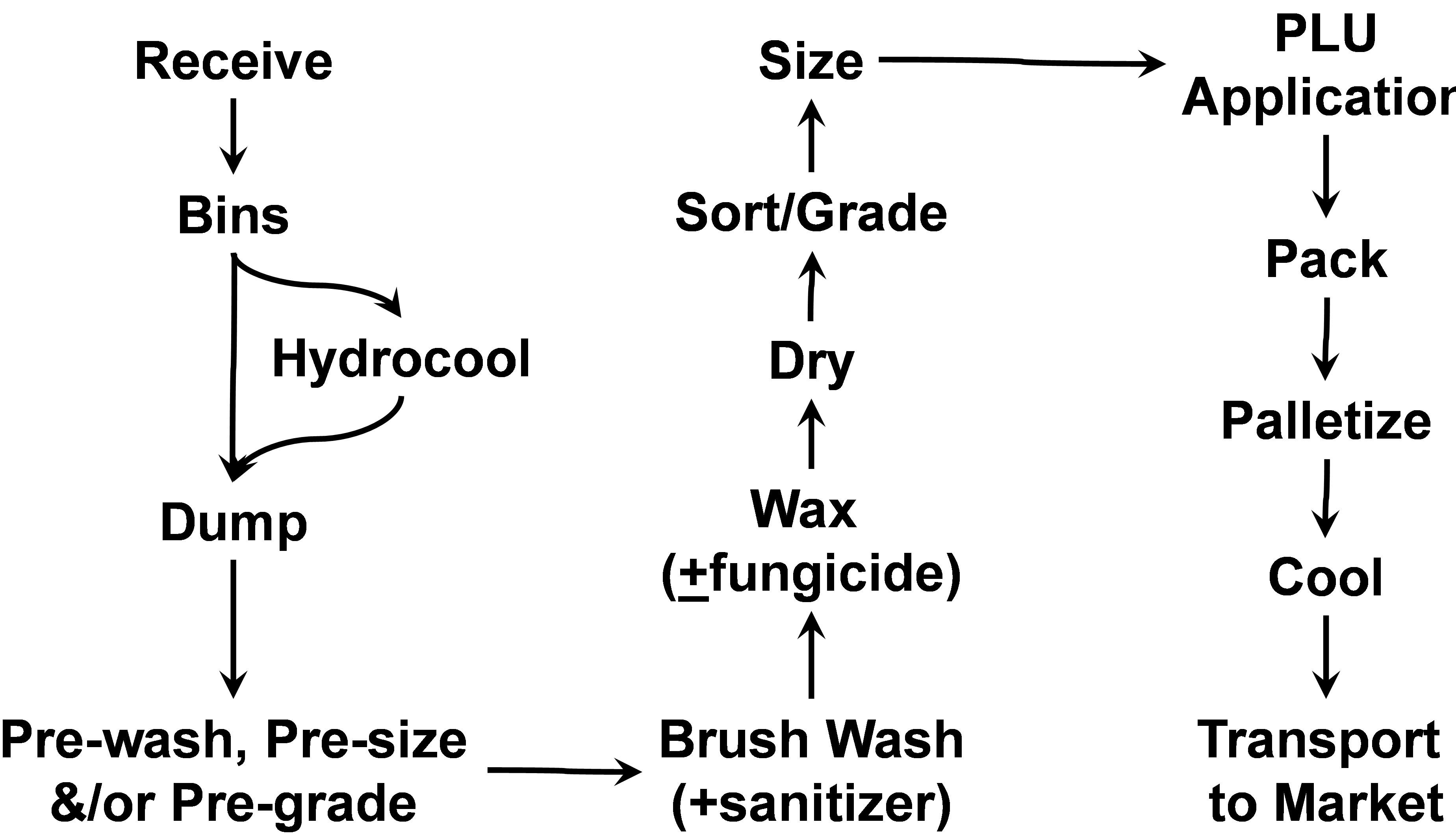
Credit: Mark A. Ritenour and Jeffrey K. Brecht, UF/IFAS
Mechanized packing is often used by high-volume stonefruit packers but requires fruit to be relatively unripe and firm when they are dumped onto the packingline to avoid bruising and other mechanical injuries. However, this system does allow for postharvest fruit treatments that are often desired by some markets, especially those markets requiring ocean transit times. Such treatments include fruit washing, brushing, waxing, and treatment with fungicides.
With this system, fruit are harvested into bags and transferred into large field bins that are transported to the packinghouse as soon as possible. The fruit should be shaded whenever possible between harvest and either precooling or dumping onto the packingline to prevent warming or sunburn of exposed fruit. Delays should be minimized between harvest and cooling to slow the fruit’s metabolism and extend shelf life.

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
Stonefruits are either precooled inside the bins immediately after arrival at the packinghouse (using a hydrocooler or forced-air cooler), or the fruit are packed within 24 hours and then cooled within the packages (using a forced-air cooler). To avoid re-warming, pre-cooled fruit need to be moved through the packingline quickly, especially if the line is not in a closed and air-conditioned building.

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Jeffrey K. Brecht, UF/IFAS
In smaller operations that need to apply certain fruit treatments but also handle riper (softer) fruit, fruit may be picked into buckets or totes, which are stacked inside of bins or placed on trailers for transport to the packinghouse. The fruit are dumped onto a low-volume packingline that is designed with greater care to minimize mechanical injuries while allowing the required fruit treatments (e.g., washing and wax/fungicide application). Such a system reduces the potential for mechanical injury of the more mature and softer fruit, while still allowing for the postharvest treatments.
Stonefruits are usually dry-dumped onto the packingline where larger debris and obviously unmarketable fruit are immediately removed. The fruit may then be washed with chlorinated water. Wet brushes used for cleaning also help remove the “fuzz” (trichomes) on the surface of peaches. Trichomes are single-celled extensions of epidermal cells, which, along with the other epidermal cells, are naturally coated with a thin layer of cutin; a material that inhibits water transmission and loss. Removing the fuzz breaks open the trichomes and enhances water loss. Visible shriveling will occur if peaches lose as little as 5% of their water content after harvest. Even though the rate of water loss varies among cultivars, steps should be taken to minimize water loss from all stonefruit. To help with this, the fruit are usually coated with a water-emulsifiable wax. Such coatings may be comprised of mineral oil, vegetable oil, or various edible coatings; mineral oils usually do the best at reducing water loss and edible coatings the worst. In addition, fungicides may be added to the coating to help control postharvest decay.

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Jeffrey K. Brecht, UF/IFAS
Sufficient area of the packingline should be devoted to grading activities. The length of the line devoted to grading will depend on the condition of the arriving fruit. The grading process removes unmarketable fruit with visible defects, such as injuries or decay, but may also be used to divert well-colored fruit to a higher-maturity pack. Mechanical sizing equipment may separate fruit based on either volume or weight and must be flexible enough to handle fruit representing a broad range of sizes. Equipment is also available that uses near infrared (NIR) light to evaluate internal sugar content at commercial line speeds (i.e., 10 fruit per second), but such technology does not appear to have been adopted much by the industry at this time. Price look up (PLU) labels are attached to each fruit by equipment that is usually installed on the sizing conveyor lines.

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
Depending on firmness, the fruit may either be place packed by hand onto trays (usually either one or two layers), or it may be mechanically packed into cartons using volume-fill packing. “Tree-ripe” fruit or fruit from softer cultivars are often packed into single-layer trays; slightly firmer fruit are packed into double-layer trays; and firm fruit are volume-fill packed. In some cases, packers are assisted by a conveyor belt moving a continuous line of trays as fruit are delivered over the top; the packers control the speed of the conveyor and position the fruit into the individual fruit cups. Fruit may also be packed into small plastic bags or plastic clamshell containers depending on fruit firmness and market requirements.

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
If stonefruits are not precooled before packing, they are usually forced-air cooled in the palletized trays or cartons before shipment. Rapid precooling is important not only for extending shelf life and slowing decay development, but also for minimizing water loss. Once the fruit are cooled, keep an unbroken cold chain with high relative humidity to minimize water loss and maximize quality retention during marketing.

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
Good cleaning and sanitation practices for the equipment and facilities are critical to minimize postharvest decay and as part of the operation’s food safety program. Dirty conditions often cause fruit abrasion, which enhances water loss, promotes infection by decay and pathogenic organisms, and may promote the development of fruit disorders such as “inking” (see Chapter 10, Postharvest Stonefruit Defects and Disorders).
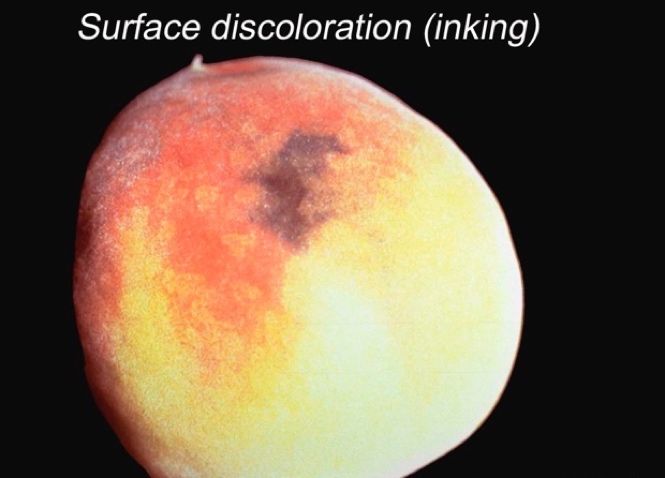
Credit: Carlos Crisosto, UC Postharvest Technology Center
Ranch Packing
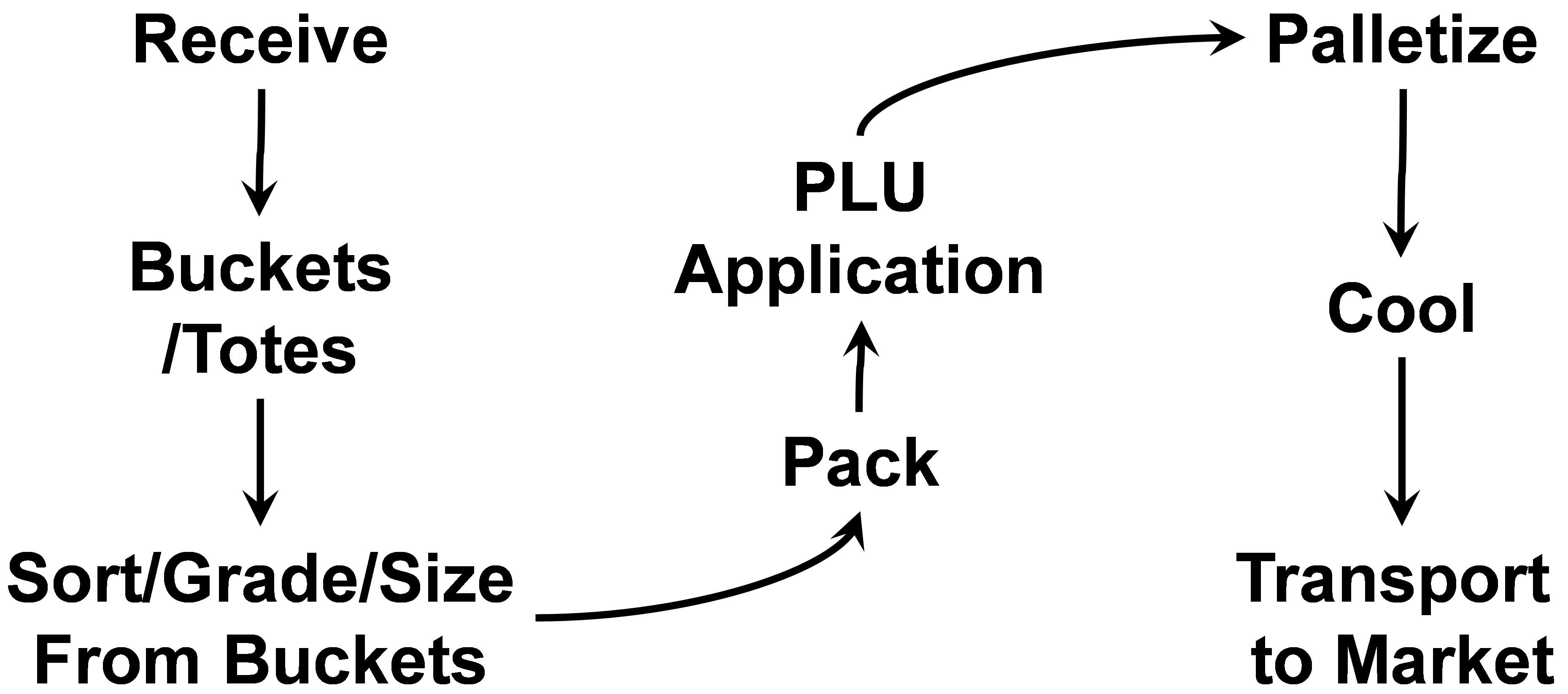
Credit: Mark A. Ritenour and Jeffrey K. Brecht, UF/IFAS
“Tree ripe” fruit that are softer but of high quality are often “ranch” or field packed near the production site. Typically, such fruit are harvested into buckets or totes, loaded onto trailers, and then transported to the packing area. At the packing area, the buckets are unloaded, and the fruit are removed from the buckets or totes and inspected individually for size and defects as they are being place packed into plastic trays. Careful supervision of the grading and packing operations is required to assure consistent quality. PLU labels may then be placed on the fruit before they are cooled (usually forced-air cooling) and shipped. The advantage of this system is that the fruit are handled as little as possible to minimize mechanical injury. The disadvantage is that postharvest treatments (i.e., washing and waxing/fungicide treatment) are not possible.

Credit: Mark A. Ritenour, UF/IFAS

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS

Credit: Mark A. Ritenour, UF/IFAS
Additional Reading
Mitchell, F. G., and A. A. Kader. 1989. “Field Handling and Packing.” In Peaches, Plums, and Nectarines: Growing and Handling for Fresh Market, edited by J. H. LaRue and R. S. Johnson, 197–208. Publ. 3331. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
Thompson, J. F., E. Mitcham, and F. G. Mitchell. 2002. “Preparation for Fresh Market.” In Postharvest Technology of Horticultural Crops, edited by A. A. Kader, 67–79. Publ. 3311. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
7. Controlling Postharvest Decays of Stonefruits Grown in Florida
Jerry A. Bartz
Decay Control: Pre- and Post-Harvest Procedures
The following descriptions and recommendations are based on an evaluation of reports of postharvest treatments used in peach-growing areas of the southeastern United States. “Decay control” is a general term meaning to keep losses at levels that do not compromise profits, markets, or brand reputations. A complete absence of decay is often difficult to achieve if one is marketing ripened peaches. Problems in eastern US production areas are somewhat different from those in the western areas of the country primarily due to climate. Similarly, problems in the northern portion of the eastern US production area are often different from those in the southern portion. This analysis will be focused on the more southern portion of the eastern US production area. Fruit decay is defined as a relatively rapid colonization of tissues leading to a generalized collapse. Pathogens causing peach fruit decays are all fungal. In general terms, measures used to control fruit decays can be grouped into management areas: i) orchard; ii) harvest; iii) handling and packing; iv) storage and sales.
Fruit Decays That Are Likely to Develop among Florida-Grown Peaches
Brown rot (Monilinia spp) is the most potentially damaging fruit decay in Florida-grown peaches (Sarkhosh et al. 2020). Trees and fruit are most vulnerable to infection during bloom periods, and the last 2 to 3 weeks prior to harvest are critical. Disease outbreaks are highly likely if blowing rainfall, freeze events, or storms occur during these developmental periods (Richie 2000). With late-maturing peaches, the beginning of summer rainfall leads to brown rot outbreaks among fruit beginning to color or approaching harvest. Additional fruit decays include Rhizopus and Gilbertella rots, sour rot, and gray mold rot. Decays likely to be observed after harvest (postharvest decays) include brown rot, Rhizopus rot, and Gilbertella rot.
Brown Rot
Cause: Several Monilinia spp. with the most common being M. fructicola. World-wide distribution.
Infection sites: Initially, flowers become infected during wet bloom periods. The pathogen grows from the blighted flower into the peduncle and then twig (Ritchie 2005). This leads to a necrosis of woody tissues and the formation of a canker. Technically, this manifestation of brown rot is not decay; however, blighted flowers and associated cankers produce high populations of the pathogen, which then infect fruit, leading to decay. Wounds that enable infection by Monilinia spp. include frost or freeze damage, insect feeding damage, hail damage, scrapes or punctures by branches and twigs during high winds, harvest injuries, and road vibration bruises during movement of harvested fruit to the packing area (Richie 2000). Packingline abrasion of fruit surfaces has also been observed (Rushing and Taylor 2002). Clusters of wild plums have become infected by fruit-to-fruit spread, but such clusters are usually not encountered in peach orchards. However, fruit-to-fruit spread can occur postharvest among peaches in bins or containers.
Signs and symptoms: Blossoms turn dark brown and necrotic, leading to a necrosis (dead tissues) that spreads into the stem and occasionally further into the twig, leading to a stem canker (Figures 7-1, 7-2, 7-3, and 7-4).

Credit: Jerry Bartz, UF/IFAS
Cankers may enlarge until stems are girdled, at which time shoot tips distal to the lesion become necrotic. Gum often oozes out of cankers. Light-brown tufts of fungal growth and sporulation appear over enlarging infections.
Small brown spots may develop on young fruit or around wounds. These spots enlarge as fruit mature and particularly as they ripen.

Credit: D. Huff and Ali Sarkhosh, UF/IFAS
Brown to tan lesions on ripening fruit enlarge rapidly and are accompanied by profuse sporulation. Skin over lesions is relatively firm—does not slip off. Fruit-to-fruit spread occurs among harvested fruit in bins or cartons, leading to a “nest” of decaying fruit and fungal growth.

Credit: Jerry A. Bartz, UF/IFAS (top) and Yuru Chang, UF/UFAS (bottom)
Prior to harvest and ripening, fruit may have hidden or latent quiescent (dormant or inactive) infections. These infections become active as the fruit ripens and become visible brown rots on fully ripe fruit.
Infection outcomes: While blossom blight seldom becomes severe enough to jeopardize yields, fruit set is reduced and those fruit that form are likely to have latent infections (Brannen and Schnabel 2002). Blossom blight and stem cankers can lead to latent infection of green fruit although Biggs et al. (1998) noted “quiescent infections of peach and nectarine have not been reported in the eastern U. S.,” ostensibly because blossom blight is not as severe there as it is in other production areas. Infections of aborted or pruned immature peaches as well as ripe peaches left on the tree progress until the fruit dehydrates into a mass of necrotic fruit tissue and fungal tissues called a mummy (Ritchie 2005).

Credit: Phil Harmon, UF/IFAS
Mummified buttons or mature fruit as well as stem cankers are sites in which the fungus survives until the next flush of susceptible peach tissues. The number of such potential sources of inoculum affects management of the next season’s crop. High levels of inoculum coupled with storm or insect damage of maturing peach crops can lead to major losses to fruit decay. Latent infections on harvest-ready fruit are more likely when high levels of inoculum are present in the orchard. Brown rot may spread through an entire mature fruit within 48 hours of infection if temperatures favor disease development. Optimal temperatures for infection range from 72.5°F to 81.5°F (22.5°C to 27.5°C) (Biggs and Northover 1988).
Orchard, Harvest, and Postharvest Practices That Reduce Losses to Brown Rot
Sanitation in the orchard, during harvest and during postharvest handling is critical to managing brown rot. If blossoms or fruit are not exposed to spores of the pathogen, decay losses to brown rot are virtually eliminated.
- Sources and amount of inoculum must be minimized. This starts with orchard location. Orchards should not be started near areas that have potential offsite inoculum such as wild or ornamental stonefruit relatives. It has been suggested to site orchards at least ¼ mile away from uncontrolled wild plum thickets or trees (Diver and Mumma 2003).
- Carry-over inocula from the previous season must be avoided to the extent possible. Fruit should never be left on trees or on the orchard floor after a harvest season. Inoculum produced on crops that have been abandoned due to severe blossom blight or freeze injury can severely threaten new crops. Cull fruit from packing operations should never be dumped in or near an orchard.
- Fruit thinning should occur prior to pit hardening because discarded fruit with hard pits are readily colonized by the pathogen, whereas less-mature thinned fruit decompose before the fungus can turn them into mummies. If this is not possible, prune fruit into buckets and carry them out of orchard.
- Monilinia spores contaminating picking containers can germinate in wounds and initiate infections. Containers can be sanitized by steam or by being washed with chlorinated water (ca. 100 ppm, pH 6.5–7.0). These sanitizers can also be used on packingline equipment. Chlorinated water is not effective if decayed fruit residues have dried on surfaces that contact fruit. Consequently, all dried residues must be removed from fruit contact surfaces if sanitation is to successfully eradicate contamination. Additionally, cleaning contact surfaces requires those surfaces to be non-porous. Wooden surfaces are much more difficult to clean than plastic or metal, particularly if the wood is not uniformly coated with paint. Chlorinated water (ca. 100 ppm free chlorine, pH 6.5–7) is also recommended for hydrocoolers or fruit wash water. This sanitation treatment won’t eliminate latent infections or spores in wounds but will prevent the accumulation of spores in the water. Note: chlorine is an oxidizer and thus corrosive (i.e., it oxidizes metals containing iron, aluminum, etc.). It continually reacts with peach surfaces, soil, plant debris, etc. as well as metal surfaces. These reactions deplete free chlorine from the solution. Therefore, chlorine levels must be maintained if the treatment is to be effective. Additionally, free chlorine activity is affected by solution pH. The best combination of activity and limited corrosiveness is at pH 6.5 to 7.0.
- Treating the host with fungicides:
-
- Two to three applications of an approved, registered fungicide are recommended during bloom periods, often as color appears after bud break or as flowers open (Brannen and Schnabel 2002). An application is particularly warranted if a cold front or other rainfall event is predicted to pass over the orchard.
- Two to three applications of an approved, registered fungicide are recommended over the 2- to 3-week period prior to harvest (as fruit begin to color), particularly if blossom blight, stem cankers, or mummified buttons have been observed.
- A postharvest drench of fruit with an approved, registered fungicide during the packing process is recommended if fruit decays are observed during harvest or if decayed fruit are culled from the harvest during packing.
The environment in the orchard and after harvest affects brown rot development.
Warm, wet environments around blossoms and fruit encourage this disease.
- Make sure trees are planted at recommended distances from each other. Tree rows should be oriented such that prevailing winds move down the row thereby drying rainfall and morning dews more rapidly.
- Irrigation should be scheduled in the morning, mid-day, or in the middle of the night (after dew fall) so that wet periods associated with irrigation and dew are minimized.
- Trees should be “trained” and pruned so that there are ample spaces for air-movement.
- Early morning harvests generally should be avoided since moisture on fruit increases chances for inoculation as well as injury.
- Harvested fruit should be promptly moved to the packinghouse for sorting and packing.
- Harvested fruit should be promptly cooled below 45°F (<7.2°C) either by a forced-air or hydrocool system and then down to 32 °F to 33 °F (0 °C to 0.6 °C) for storage and shipment. Peach fruit should not be stored at temperatures between 36°F and 46°F (2.2°C and 7.8°C) due to sensitivity to chilling injury, which not only predisposes them to decay but also loss of quality. (Schnabel and Miller 2002)
Managing the Host to Reduce Fruit Decays
- Choose cultivars that have some resistance to Monilinia fruit decays.
- Supervise harvest operations to keep mechanical injuries at a minimum.
- Avoid harvest of cold or wet fruit. The wetness encourages infection (see above), and cold fruit of many different types are known to be more sensitive to impact injury and bruising.
- Harvest fruit at the right stage and keep it cool. Peach fruit become more susceptible to brown rot as they ripen. Consequently, making sure fruit are at the proper stage of maturity at harvest and slowing their ripening processes by cooling postharvest ensures the highest level of resistance to fruit decay.
Rhizopus Rot (Often Combined with Very Similar Gilbertella Rot)
Causal agents: Rhizopus stolonifer or Gilbertella persicaria. Both appear to have world-wide distribution. Both are more common on ripe to over-ripe fruit. Both are strict wound invaders but can move from fruit to fruit to create nests in packed fruit (Ginting and Zehr 1992).
Signs and symptoms: Initial symptoms include a slightly darkened water soaking around wounds or a distended surface over a slightly softened area that is associated with internal lesions. With either type of initial symptom, the decay enlarges rapidly and is accompanied by dark brown to black or green coarse mold growth on the lesion surface. The infected tissues are very soft and often ooze fluids that can move spores throughout cartons of bins.

Credit: UC Postharvest Technology Center
Infection Outcomes: Infected fruit quickly collapse, and nests of decaying fruit and fungal mycelium develop. Fruit flies often infest advanced decays and nests of decay. Such insects can vector spores to wounds, which then become infected. Consumers who find these decays in their peach purchases at home will be reluctant to make repeat purchases. The fluids, the mess, and the odors of these decays are suggestive of decaying garbage.
Orchard, Harvest, and Postharvest Practices That Reduce Losses to Rhizopus/Gilbertella Rots
- As with managing brown rot, managing both Rhizopus and Gilbertella rots requires effective sanitation. Both pathogens are proficient saprophytes and normally live on decaying organic matter. Inoculum sources in the orchard include abscised fruit or attached, over-ripe and often injured fruit.
- Cull fruit from packing operations should never be dumped in or near an orchard or left near a packing facility. Picking containers and field bins should be regularly cleaned and sanitized.
- Both pathogens can survive in fresh or dried debris attached to the inside of bins and containers as well as surface growth on storage room walls. A particularly egregious practice is to not clean or sanitize containers that have been moistened by decaying fruit. Containers and storage rooms can be sanitized by steam or by being washed with chlorinated water (ca. 100 ppm, pH 6.5–7.0). These sanitizers can also be used on packingline equipment. Since chlorinated water is not effective if decayed fruit residues have dried on equipment, all surfaces including storage room walls must be cleaned by applying a sanitizer.
- Chlorinated water (ca. 100 ppm free chlorine, pH 6.5–7) is also recommended for hydrocoolers or fruit wash water. As with brown rot, this sanitation treatment won’t eliminate latent infections or spores in wounds but will prevent an accumulation of spores in the water.
- Since both pathogens are strict wound-invaders, harvest-related injuries must be minimized. Avoid harvesting wet trees such as during the early morning or too soon after rainfall or irrigation.
- Harvested fruit should be promptly and gently moved to the packinghouse for sorting and packing and then promptly cooled (Schnabel and Miller 2002).
The Environment after Harvest Affects Rhizopus/Gilbertella Development
Wet, warm environments favor rapid decay development. The most important step in creating an environment that inhibits these decays is prompt and continuous refrigeration (see brown rot control for details).
Managing the Host to Reduce Fruit Decays
- Supervise harvest operations to keep mechanical injuries at a minimum. This includes minimizing road shock and vibration as fruit are moved from field to packing facility.
- Avoid harvest of cold or wet fruit (see above).
- Fruit can be treated with an approved, registered fungicide on the packingline. Such applications usually aren’t needed if the cold chain can be maintained (fruit stored, shipped, and marketed at <38 °F/3.3 °C).
- Infections often occur on overripe fruit. Thus, harvesting fruit at the appropriate stage or maturity and cooling them so they do not ripen rapidly helps to maintain the natural resistance of fruit to these decays.
Gray Mold Rot
Causal agent: Botrytis cinerea
Infection sites: Blossoms and injuries on fruit.
Signs and symptoms: Brown, somewhat firm lesions develop on fruit usually near the blossom end or at injuries. As the lesion enlarges, a blue to gray mass of spores and mycelium forms on its surface. This will slowly expand until the entire fruit has decayed.
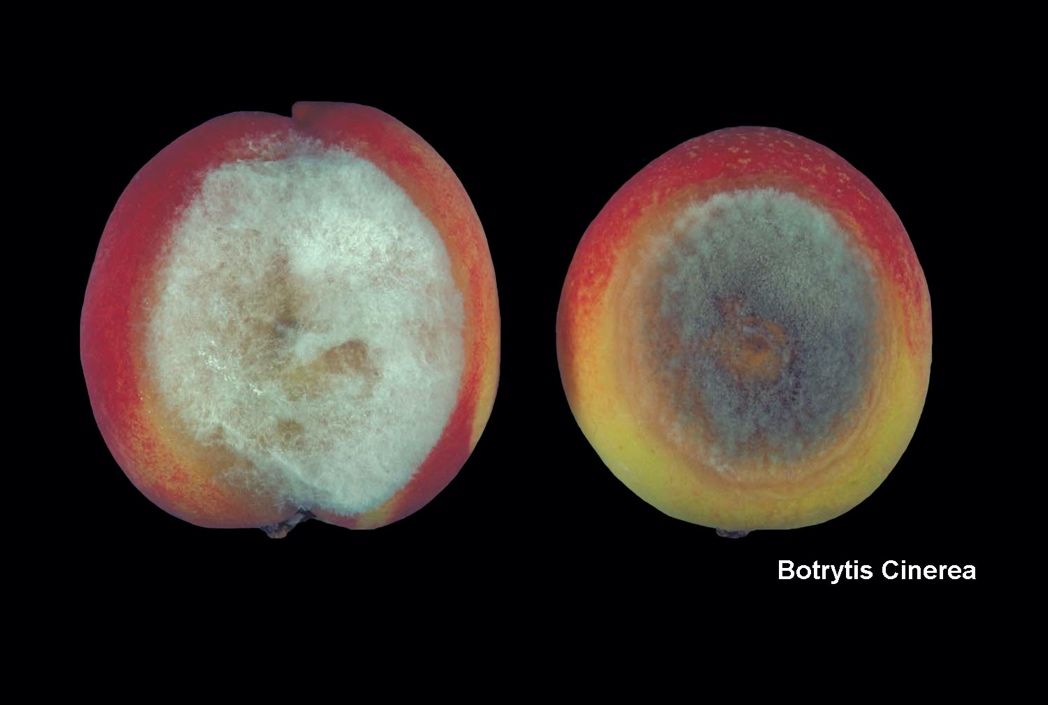
Credit: Don Edwards, U.C. Davis
Infection outcomes: Gray mold develops on blossoms and fruit damaged by freeze events, insects, and storms. Movement to undamaged fruit occurs during cool, foggy weather, which usually does not occur in the southeastern US production area but is common in coastal orchards of the western production areas (Schnabel and Miller 2002). When daytime temperatures exceed 80 °F (26.7 °C), the disease is not a concern. Postharvest gray mold will spread from fruit to fruit and the high humidity and cool temperatures in storages are favorable for infection and spread. The pathogen will grow at temperatures below the freezing point of fruit flesh. However, cold storage remains one of the best ways to control the disease.
Orchard, Harvest, and Postharvest Practices That Reduce Losses to Gray Mold Rot
- Sanitation in and around the orchard and packing facility is recommended. Removal of wild fruit trees or abandoned fruit crops such as strawberry will reduce sources of inoculum. Additionally, cull fruit should be promptly removed from packing facilities and never dumped near orchards.
- If gray mold signs or symptoms are observed in the orchard, a fungicide spray program is recommended.
- Sanitation of dump tank, flume, and wash or hydrocooler water is essential. See discussion of sanitizers under “brown rot.”
- Drench or spray bar treatment of fruit on the packingline with an approved fungicide is helpful if gray mold has been observed in the orchard at the time of harvest.
- Prompt cooling is absolutely essential (see previous discussion).
Management of Storage Environment
- Like the other molds, B. cinerea may grow on moist containers or storage room walls as well as on the fins of heat-exchangers. Blowing air will distribute spores from these growths throughout fruit stored in the facility. Consequently, these surfaces should be cleaned and sanitized regularly.
- Packed fruit should be quickly cooled by forced-air cooling or hydrocooling.
- Room cooling does not cool packed fruit uniformly or quickly. Pockets of warm fruit enable decay pathogens to colonize tissues and begin forming nests. Additionally, condensation may form on cooled fruit that become contacted by warm humid air. Wet fruit surfaces are ideal for infection by fruit decay pathogens.
Managing the Host to Reduce Fruit Decays
- As with the other fruit decays, fruit should be harvested carefully into cleaned and sanitized bins. These bins of fruit should be gently moved from orchard to packing facility. This reduces the number of potential infection sites.
- Cool fruit that are somewhat less than fully ripe and are free from injuries have a natural resistance to gray mold rot.
Other Potential Fruit Decays
Sour rot caused by Geotrichum candidum has been reported in southeastern US peaches but is relatively uncommon. Similarly, Penicillium and Alternaria molds have been isolated from decaying peaches. The key to controlling these decays as well as the ones detailed above is sanitation and prompt cooling to recommended storage temperatures. Note: G. candidum is known to readily spread among tomato fruit by fruit flies. Expect this to occur with peaches as well. Thus, it is absolutely essential to move cull fruit and fruit debris away from packing facilities.
What is sanitary design?
According to Rushing (2010), sanitary design simply means that equipment and packinglines are designed to enable proper cleaning and inspection between uses. Packing areas are set up so that any equipment, tables, and other surfaces:
- Can be accessed for inspection. Allow for room between equipment and walls, so that all parts of the packingline can be monitored for buildup of dirt and debris. Shields can be removed for further inspection.
- Are constructed from materials that can be properly cleaned and sanitized. Attributes of sanitary construction include surfaces that are:
- hard, impervious and nonabsorbent,
- easily cleanable,
- smooth,
- resistant to wear and corrosion,
- able to withstand the action of cleaning and compounds, and
- light colored (so that dirt and buildup can be easily spotted).
Materials such as wood, sponges, and foam are almost impossible to clean and sanitize when compared to metal and stainless steel. If possible, cover wood and foam bumpers on packing equipment with vinyl or another material that can be cleaned. Wooden tables can also be covered with plastic or vinyl (even tablecloths), to allow for cleaning and sanitization.
Additional Reading
Biggs, A. R., and J. Northover. 1988. “Influence of Temperature and Wetness on Infection of Peach and Sweet Cherry Fruits by Monilinia fructicola.” Phytopathology 78:1352–1356. https://doi.org/10.1094/Phyto-78-1352
Brannen, P. M., and G. Schnabel. 2002. “Management of Preharvest and Postharvest Brown Rot of Peach in the Southeast.” Southeastern Regional Peach Newsletter. May. p. 5.
Diver, S., and T. Mumma. 2003. “Organic and Low-Spray Peach Production.” www.attra.ncat.org
Ginting, C., and E. I. Zehr. 1992. Gilbertella Rot of Peaches Caused by G. persicaria in South Carolina.” Plant Disease 76:753. https://doi.org/10.1094/PD-76-0753D
Richie, D. F. 2000. “Brown Rot of Stone Fruits.” The Plant Health Instructor. https://doi.org/10.1094/PHI-I-2000-1025-01
Rushing, J. W. 2010. “Facilities and Equipment Cleaning and Sanitation.” In Improving the Safety and Quality of Fresh Fruits and Vegetables: A Training Manual for Trainers. Joint Institute for Food Safety and Applied Nutrition. p. 203.
Rushing, J. W., and K. C. Taylor. 2002. Post-Harvest Management: “Peach Skin Discoloration and Water Quality Management.” Southeastern Peach Growers Handbook. University of Georgia College of Agric. & Environ. Sci., Athens, GA. p. 121–126.
Sarkhosh, A., S. Shahkoomahally, L. M. Richmond-Cosie, and P. Harmon. 2020. “Peach Brown Rot.” HS1357 EDIS 2020 (1). https://doi.org/10.32473/edis-hs1357-2020
Schnabel, G., and R. W. Miller. 2002. “Post-Harvest Decay.” In Horton, D. and Johnson, D. (eds), Southeastern Peach Growers’ Handbook. University of Georgia College of Agric. & Environ. Sci., Athens, GA. p. 137–141.
8. Managing the Postharvest Environment – Best Practices for Stonefruit Precooling, Storage, and Shipping
Steven A. Sargent, Jeffrey K. Brecht, and Mark A. Ritenour
Importance of Cooling and Temperature Management
As detailed in previous chapters, maximizing the postharvest quality and shelf life of perishable fruits like peaches, nectarines, and plums, called “stonefruit,” is challenging. Critical first steps include harvesting at proper ripeness stage and minimizing mechanical injury during harvest and handling. Then, maximum shelf life is made possible by rapidly and thoroughly cooling prior to shipping and by maintaining recommended temperature and relative humidity (RH) conditions thereafter.
Timely cooling is critical because, under ambient conditions, respiration and ripening are accelerated, which result in softening, overripening, and loss of nutrients. Moisture loss is also rapid under ambient conditions, with shriveling becoming apparent in as few as 3 days at 70 °F (21.1 °C) and low RH (50%). Cooling stonefruit as soon as possible after harvest and holding them at near 32 °F (0 °C) and 90% to 95% RH slows these processes and extends shelf life up to 6 weeks. Note that stonefruits are susceptible to chilling injury (internal breakdown) when held at 36 °F to 50 °F (2.2 to 10 °C) for extended periods; therefore, they should be stored in the 32 °F to 34 °F (0 °C to 1.1 °C) temperature range if storage periods will be lengthy.
Cooling also slows development of postharvest decays, notably brown rot (Monilinia fructicola). For more information on stonefruit decay, see Chapter 7 in this handbook and Peach Brown Rot, by Sarkhosh et al., 2020 (https://edis.ifas.ufl.edu/hs1357).
For roadside marketing, short times from harvest to purchase reduce the need to cool the fruit to near freezing temperatures. Fruit quality can be maintained for 2 to 3 days by cooling to 45 °F to 50 °F (7.2 °C to 10 °C) in a refrigerator or refrigerated storage room while providing consumers with plastic bags (grocery store produce bags) helps reduce water loss by keeping high RH (90% to 95%).
Principles for Effective Cooling
- Cooling time and 7/8 cooling
- The three Ts: time, temperature, turbulence
- Efficacy of cooling medium: heat capacity of air vs. water
- Cooling efficiency: effect of venting, and pallet openings on cooling rate
Effective cooling involves lowering the pulp temperature of the fruit to the desired level using a cooling method that is compatible with the crop and that efficiently cools it. Use of the wrong cooling method could result in inadequate cooling or even cause damage to the crop. If the cooling method does not cool efficiently, the process will take longer to reach the desired final pulp temperature, resulting in non-uniform final pulp temperatures within the load. Depending upon the handling scenario, stonefruit can either be partially or completely cooled the day of harvest for same-day packing or packing the following day. Cooling the fruit prior to packing reduces mechanical damage during packing operations. Since stonefruit tolerate contact with both cold air and water, forced-air cooling and hydrocooling are the recommended cooling methods.
The goal of commercial cooling is to remove 7/8 of the field heat during the cooling process; in other words, the fruit are cooled to 7/8 of the difference between the incoming pulp temperature and the cooling air or water temperature (Figure 8-1). The remaining 1/8 of the pulp temperature is removed during storage.
Determination of the 7/8 cooling time can be easily understood by studying an idealized cooling curve (Figure 8-1). In this example, fruit with an initial pulp temperature of 68 °F (20 °C) are placed in a cold room at 32 °F (0 °C). The target temperature for 7/8 cooling is calculated as follows:
Subtract the cold room air temperature from the initial pulp temperature (68 °F – 32 °F = 36 °F); this is the total possible pulp temperature drop during cooling.
Multiply this total temperature drop by 7/8 (36 °F x 7/8 = 31.5 °F); this is the 7/8 temperature drop.
Subtract the 7/8 temperature drop from the initial pulp temperature (68 °F - 31.5 °F = 36.5 °F); this is the fruit temperature when 7/8 cooling is complete and the point at which the crop is transferred to the storage room, where the remaining 1/8 of the field heat will be removed.
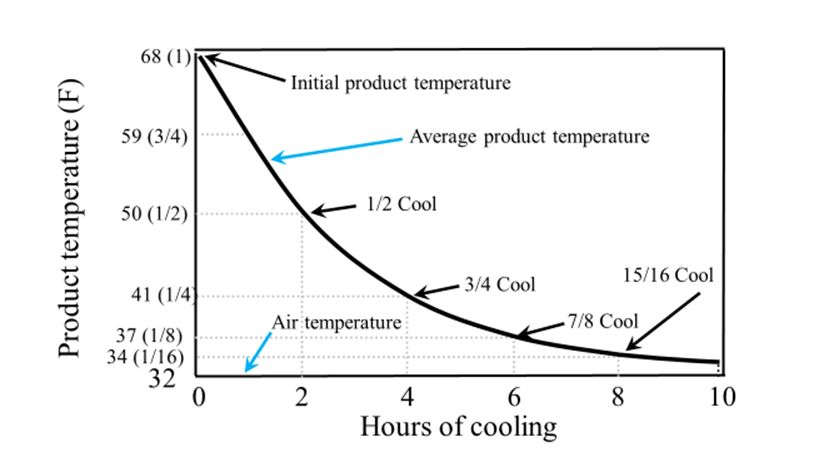
Credit: Adapted from Thompson et al. 2008
Returning to Figure 8-1, the curve shows the pulp temperature decline during 10 hours of cooling. Notice that the cooling rate is fastest during the first 2 hours, when the pulp temperature dropped by 18 °F (10 °C), from 68 °F to 50 °F (20 °C to 10 °C). This is known as the “1/2 cooling time” since the pulp temperature decreases by one half (18 degrees is one half of the 36-degree difference between the initial pulp temperature of 68 °F and the cold room temperature of 32 °F).
Next, notice that the cooling rate from 2 to 4 hours slows relative to the first 2 hours so that the pulp temperature decreases by only 9 °F (5 °C) to 41 °F (5 °C), half as much as in the first 2 hours; 4 hours in this case is the 3/4 cooling time. From 4 to 6 hours of cooling, the pulp temperature decreases by only 4.5 °F (2.5 °C) to 36.5 °F (2.5 °C); thus, the 7/8 cooling time is 6 hours under these conditions. At this point, 7/8 cooling is complete, and the crop should be moved to a storage cold room at 32 °F (0 °C) where the remaining 1/8 of the pulp temperature would be lowered to reach the air temperature of 32 °F.
Note: Figure 8-1 shows the cooling medium temperature being maintained throughout the cooling process. In fact, in commercial cooling operations in which the field heat is removed from the fruit more rapidly than with room cooling (i.e., forced-air cooling and hydrocooling), the cooling medium temperature often increases during the early part of the cooling process when the bulk of the field heat is being removed from the fruit. This is because the heat load temporarily exceeds the refrigeration capacity; however, the cooling medium temperature usually returns to its original set point later in the cooling process. Experienced operators learn to follow the cooling medium temperature to know how the cooling process is proceeding.
Cooling efficiency is defined by three principles, and these can be easily remembered as the Three Ts: temperature, time, and turbulence. The effect of each T on the time to reach 7/8 cooling is described below.
Temperature refers to the temperature of the cooling medium; for stonefruit, this is air or water, and efficiencies of the three cooling methods are shown in Figure 8-2. The cooling system must be designed with sufficient refrigeration capacity to maintain the temperature of the cooling medium during the entire cooling process. If not, the air or water temperature will gradually increase during cooling, extending the cooling time and preventing the pulp temperature from being reduced by 7/8. A temperature probe is useful to track the fruit pulp temperature during cooling (Figure 8-3).
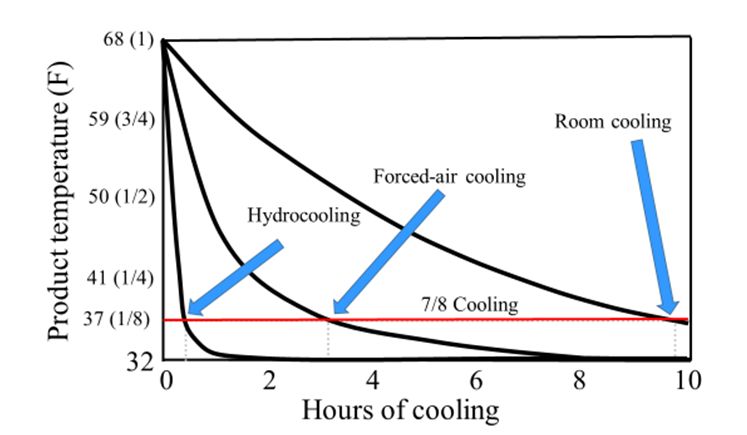
Credit: Adapted from Thompson et al. 2008

Credit: Jeffrey K. Brecht, UF/IFAS
Time refers to the length of time the crop is exposed to the air or water cooling medium. Once a cooling process has been documented, the 7/8 cooling time can be longer or shorter depending on the incoming crop pulp temperature.
Turbulence refers to the mixing or close contact of the cooling medium (water or air) with the crop. The fastest cooling occurs when the crop is in direct contact with the cooling air or water, as in the case of loose fruit being immersed in cold water or being exposed to cold air in a single layer on a conveyor. Cooling in field bins or field lugs or in shipping trays or cartons on pallets restricts air or water flow, slowing down the cooling process.
Cooling Methods for Stonefruits
There are three recommended methods for cooling stonefruits: Room cooling, forced-air cooling, and hydrocooling. Small operations may choose to use room cooling, in which freshly picked fruit in small containers are placed in a refrigerated room, preferably in a single layer. This is the slowest method and should only be used for short turn-around times and quick sales within a day or so of harvest. During room cooling (Figure 8-4), the crop loses significantly more moisture (fresh weight) than during the faster cooling methods, forced-air cooling and hydrocooling.

Credit: Jeffrey K. Brecht, UF/IFAS
Having access to a cold room allows the possibility to upgrade to forced-air cooling, also known as pressure cooling and tunnel cooling. This method accommodates the largest range of crops and can achieve 7/8 cooling three to four times faster than can room cooling (Figure 8-1). Forced-air cooling employs a high-capacity fan mounted in a false wall that separates the cold room from the plenum (an air space between that wall and the outer wall of the cold room) to pull the cold room air quickly through vent openings and across the fruit in the containers (Figure 8-5). To ensure that the room air temperature remains constant during cooling, the refrigeration system in the cold room must be designed with sufficient capacity to accommodate the faster removal of heat. In practice, however, cost may dictate that a smaller than optimum refrigeration system be used, somewhat lengthening the cooling time. As mentioned above under Turbulence, forced-air cooling efficiency decreases as the amount of packaging increases. Forced-air cooling can be applied to fruit in single or palletized containers (field lugs or cartons) and in field bins.

Credit: Jeffrey K. Brecht, UF/IFAS
To achieve the most efficient forced-air cooling, the side vents in the containers should account for about 5% of the area of the side face. Also, all other air openings in the forced-air cooling tunnel should be blocked, including spaces between pallets, under the pallets, and at the front of the tunnel, where the pallet meets the plenum (Figure 8-6). If those openings are not blocked, the air will pass through them, short-circuiting the vent openings in the containers.

Credit: Mark A. Ritenour, UF/IFAS
It seems logical that forced-air cooling might increase water loss from fruits, but the reality is that faster cooling reduces the temperature difference between air and fruit faster, reducing overall water loss. Thus, water loss is actually less for forced-air cooling than for room cooling. Stonefruits retain more moisture during both cooling and storage when the room RH is greater than 85%, and the use of room humidifiers is important for maintaining consistent, adequate cold room RH (Figure 8-7).

Credit: Jeffrey K. Brecht, UF/IFAS
Hydrocooling is the fastest cooling method for stonefruit. The heat capacity of water is many times higher than that for air; because of that, hydrocooling cools two to three times faster than forced-air cooling while keeping the fruit hydrated (Figure 8-2). There are two types of hydrocoolers. Shower hydrocoolers cool the fruit either in a batch underneath a shower of recirculated cold water (Figure 8-8) or by conveying the fruit though a tunnel underneath the shower (Figure 8-9). Immersion hydrocoolers are less common, and typically are used as a flume in which individual or containerized fruit are carried through the water for the time necessary to achieve 7/8 cooling. Hydrocooling can be accomplished while the fruit are in single or palletized field lugs or in field bins.
Although it is the fastest of the three methods, hydrocooling requires significantly more management because the water must be kept sanitary. Since the chilled water is recirculated, it must be continuously strained, and a food-grade sanitizer product used to reduce microbe levels and avoid cross-contaminating clean fruit with decay or human pathogens from contaminated fruit. Chlorine products and peroxyacetic acid are the most commonly used sanitizers; each has its own benefits and drawbacks (Ritenour et al. 2002).

Credit: Jeffrey K. Brecht, UF/IFAS

Credit: Jeffrey K. Brecht, UF/IFAS
To achieve most efficient hydrocooling, the water must completely cover the top surface of the palletized containers, as shown in Figures 8-8 and 8-9. The containers must have adequately sized bottom openings (and top openings if covered) so that the chilled water can easily percolate down through the containers and come into contact with the fruit.
Temperature Management during Storage and Shipping
Once stonefruits are cooled, it is critical to “maintain the cold chain” by keeping them continuously at the desired, optimum temperature during storage and shipping. Maintaining the cold chain ensures that fruit quality will be extended as long as possible. From a cost perspective, if the cold chain is maintained, the crop won’t need to be re-cooled to the optimum temperature later; in fact, re-cooling within the distribution system after warming occurs is realistically impossible. When re-warming does occur, such as when the cold fruit are set on an unrefrigerated loading dock or loading into a warm refrigerated trailer, condensation (“sweating”) will quickly develop. Once returned to the cold environment, the free water on the fruit doesn’t evaporate quickly and will promote growth of decay pathogens. Therefore, maintaining the fruit at the desired, optimum temperature is critical.
Ethylene Management
Presence of ethylene in the environment around harvested peaches can accelerate their ripening, which is usually undesirable. However, one of the benefits of good temperature management is that low temperatures slow down the rate of ethylene production by the peaches, slowing down their ripening as well. In some situations, such as during extended storage or for longer shipping durations, it can be useful to take steps to further reduce ethylene in the environment. Although management of ventilation can reduce ethylene levels, ethylene reduction is most commonly accomplished by using ethylene scrubbers like potassium permanganate-impregnated pellets (Purafil®, Ethylene Control, etc.) that react with ethylene and oxidize it to form carbon dioxide and water, or palladium-based (It’s Fresh!) scrubbers that strongly bind ethylene. Scrubbers that are based on oxidation of ethylene via ozone generators or ultraviolet (UV) energy sources, which produce ozone and other reactive oxygen species from oxygen in the air, are also available to remove ethylene from the storage environment.
Modified and Controlled Atmospheres (MA and CA) and Modified Atmosphere Packaging (MAP)
Maintaining fruits and vegetables in atmospheres with reduced oxygen (typically in the range of 2%–5%) and sometimes with elevated carbon dioxide (0%–20%) has been shown to be an effective supplement to good temperature management in slowing ripening and quality deterioration during the postharvest period. These atmospheres extend the product’s postharvest life by inhibiting respiration, which supplies the energy to carry out metabolic reactions, and by inhibiting ethylene synthesis and action for climacteric fruit like most stonefruit, which inhibits their ripening process.
The technology is called modified atmosphere, or MA, if the atmosphere is generated by the fruit’s own respiration and maintained by a certain amount of designed, restricted gas exchange into and out of the storage space. If the gases are purposefully generated and maintained in the storage room or marine container by electronic control systems, it is called controlled atmosphere storage, or CA storage. Packaging can also be designed to modify the oxygen and carbon dioxide levels around the products, and this is called modified atmosphere packaging or MAP. Semipermeable films or microporous patches are selected to match the fruit’s respiration rate and the package dimensions so that the package atmosphere equilibrates at a beneficial combination of oxygen and carbon dioxide. Ethylene scrubbing is often a key component of MA/CA/MAP systems for climacteric fruits in order to further inhibit their ripening.
Peaches respond favorably to MA/CA/MAP, but the technology has been found to be unnecessary for domestic distribution in the United States because temperature control alone almost always provides sufficient shelf life. Peaches intended for long-term storage or international transport have been found to respond best to atmospheres in CA, MA, or MAP of about 2% O2 plus 3%–5% CO2 at 32 °F (0 °C). Long exposure times at even more extreme atmospheres (lower O2 and higher CO2) can induce fermented off flavors, failure to ripen, and skin and flesh browning. However, an atmosphere of 6% O2 plus 17% CO2 has been shown to reduce internal breakdown (chilling injury) of peaches during 2- to 3-week trans-Pacific shipments. With such a wide variability in potential atmospheres, it should be clear that the best atmosphere to use must be determined through testing prior to any commercial application.
Additional Reading
Brecht, J. K., S. A. Sargent, P. E. Brecht, J. Saenz, and L. Rodowick, edited by J. K. Brecht. 2019. “Protecting Perishable Foods During Transport by Truck or Rail.” HS1328. EDIS 2019 (2). https://doi.org/10.32473/edis-hs1328-2019
Gross, K. C., C. Y. Wang, and M. E. Saltveit. 2016. The Commercial Storage of Fruits, Vegetables, and Florist and Nursery Crops, U.S. Department of Agriculture, Agricultural Research Service, Beltsville Area, Agriculture Handbook No. 66. https://www.ars.usda.gov/ARSUserFiles/oc/np/CommercialStorage/CommercialStorage.pdf
Kader, A. A. (ed.). 2002. Postharvest Technology of Horticultural Crops. Publ. 3311. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
Ritenour, M. A., S. A. Sargent, and J. A. Bartz. 2002. “Chlorine Use in Produce Packing Lines.” HS761/CH160. EDIS 2002 (7). https://doi.org/10.32473/edis-ch160-2002
Thompson, J. F., F. G. Mitchell, T. R. Rumsey, R. F. Kasmire, and C. H. Crisosto. 2008. “Commercial Cooling of Fruits, Vegetables, and Flowers. Publ. 21567. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
9. Postharvest Stonefruit Defects and Disorders
Jeffrey K. Brecht and Mark A. Ritenour
Stonefruit grown in Florida, including peaches, nectarines, and plums, can exhibit a number of defects and are susceptible to several physiological disorders that develop after harvest. In some cases, the defects and disorders are initiated before harvest, but the symptoms either don’t become apparent until after harvest or must be graded out to meet grade standard requirements. Other physiological disorders are caused by the postharvest conditions to which the fruit are exposed. The symptoms of these disorders may develop during storage or transport, or they may become apparent only later, at the retail level or when the fruit are in the consumers’ possession.
The following disorders are classified as disorders of either preharvest or postharvest origin and are arranged alphabetically within each of the two groups.
Disorders of Preharvest Origin
Abrasion Injuries (Russeting, Scabbing)
Abrasion of fruit on the tree from rubbing against leaves, branches, and other fruit can cause mechanical injury to the peel that results in scabbing or russeting as the fruit heals the damaged area. These healed injuries must be graded out on the packingline because they are counted as defects against the standards for all grades of stonefruit. Abrasion of fruit on the tree can be minimized or avoided when thinning the developing fruit by leaving sufficient spacing so that the fruit do not contact branches and other fruit.

Credit: Dustin Huff, UF/IFAS
Split Pit
Split pit describes a condition in which the two halves of the stone or pit fail to fuse together. This leaves the interior of the pit and the seed exposed at the stem end of the fruit. Decay commonly develops within the split pit, if not on the tree, then during postharvest handling. Fruit with split pits may not be easily identifiable while they are being picked, but workers should be trained to be observant and avoid collecting fruit with this defect, because they are counted as defects against the standards for all grades of stonefruit and because of the likelihood of decay.

Credit: Dustin Huff, UF/IFAS
Sunburn
Direct exposure of the surface of stonefruit to sunlight can injure and eventually kill the exposed surface tissue due to the associated heat and photooxidation. The injured area becomes sunken and discolored. Direct exposure to sunlight can increase the fruit surface temperature by as much as 20 °F (11 °C) above the shaded air temperature and the fruit surface tissues can be killed with prolonged exposure to temperatures of around 113°F (45°C) and above, or 10 minutes exposure to around 125 °F (52 °C). Poor leaf coverage in the orchard that allows all-day sun exposure can result in sunburn, but harvested fruit are particularly susceptible if the fruit that are accumulated in bins are not protected from the sun while in the orchard and while in transit to the packinghouse. Sunburn is counted as a defect against the standards for all grades of stonefruit. In addition, Alternaria fungus (Alternaria rot), a saprophyte that typically grows on dead plant tissue, as well as Monilinia (brown rot), can develop on the injured areas of sunburned fruit, either while the fruit are on the tree or during the postharvest period.

Credit: Jeffrey K. Brecht, UF/IFAS
Disorders of Postharvest Origin
Bruising and Other Physical Injuries
Peaches with the traditional melting-flesh trait are quite susceptible to bruising, especially once they begin to ripen. Fortunately, the University of Florida Institute of Food and Agricultural Sciences (UF/IFAS) Stonefruit Breeding Program has developed many new fresh-market peach and nectarine cultivars with the non-melting-flesh trait. The UF/IFAS Stonefruit Breeding Program cultivars, largely because they have non-melting flesh and are therefore resistant to bruising, have allowed a tree-ripe stonefruit industry to grow and become established in Florida. Bruising can occur due to either impacts or compression when the force is sufficient to crush the affected tissue and cause cells to break open and release their juice. Impact bruising can result from dropping the fruit, which can occur during harvesting or on the packingline, or when fruit moving too quickly on a packingline roll into a divider or wall. Compression bruising usually occurs in field bins, especially if the bins are overfilled and then stacked, but also when the bins being used are simply too deep so that the weight of fruit above the bottom layer is enough to crush the lower fruit.
Minimizing bruising is important because visible bruises count as serious defects in stonefruit grade standards and thus negatively impact marketability. To avoid impact bruising during harvesting, harvesters must be trained to gently transfer the fruit into their picking containers and from the picking containers into field bins. Damaging drops on packinglines can be avoided by using a wet dump or devices that meter the fruit as they are being poured out of field bins onto the packingline, then by designing the packingline itself to minimize the drop height of any transfers between pieces of equipment and into the shipping carton if using a volume-fill procedure.
Damaging impacts against walls and dividers on a packingline are related to the speed at which fruit are moving when they hit the wall or divider. Avoiding those impacts can be accomplished by adjusting the speed of rollers and belt conveyors and by managing the packingline fill. Keeping the packingline surface fully covered by fruit reduces the possibility that individual fruit can build up momentum and impact a surface with sufficient force to cause bruising. Applying padding such as closed-cell foam to packingline surfaces where the fruit are prone to impacts also reduces bruising by spreading out the impact force.
Stonefruits are also quite susceptible to abrasion injuries, with even minor abrasion that merely breaks the trichomes (“fuzz”) on the fruit surface leading to increased water loss and shriveling. More serious abrasions cause the damaged area to discolor due to oxidative browning or inking if heavy metals are present in water that contacts the fruit. Abrasion mostly results from fruit rubbing against each other or against container surfaces due to vibration. This mainly occurs during transport of fruit from the orchard to the packinghouse and during highway transport of packed fruit, although abrasion occurs as fruit pass over the packingline, as well. Avoiding abrasion injury begins by reducing vibrations, such as by installing air-suspension systems on the axles of field and highway trucks. It is also recommended that farm roads should be graded to reduce vibrations, reduced transport speeds between the orchard and the packinghouse should be strictly enforced, and rough roads should generally be avoided.
It is also possible to minimize abrasion damage due to vibrations by taking steps to immobilize the fruit. Field bins can be fitted with foam under a solid lid, and foam inserts can be placed in shipping cartons to immobilize the fruit. Lining wooden field bins with plastic reduces abrasion caused by the fruit rubbing against rough wood surfaces. Stonefruits are often placed in plastic trays with molded cups within the shipping carton that separate the individual fruit to prevent abrasion from fruit-to-fruit contact. Since even incidental contact with rollers and brush beds on a packingline can damage trichomes, peaches are commonly coated with various fruit waxes to reduce water loss during subsequent storage and shipping.
Physical injuries to stonefruit like cuts and punctures can occur at any step from harvesting through consumption. Training workers to be careful in handling the fruit during harvest should avoid most cuts and punctures. When workers in the field and in the packinghouse wear gloves for handling fruit (primarily due to food safety concerns), the chance of fingernail punctures is eliminated. Stonefruit packinglines must of course be designed and maintained to eliminate or cover any sharp corners and edges that could conceivably injure the fruit.

Credit: Dustin Huff, UF/IFAS
Chilling Injury (Internal Breakdown)
Stonefruit can be injured by exposure to temperatures after harvest of less than around 50 °F (10 °C), with the rate of development and severity of the injury influenced by both the temperature and the duration of exposure. This chilling injury is commonly called “internal breakdown” (IB), and the symptoms include internal pit cavity and flesh browning; flesh that is dry (not juicy), mealy, or leathery, depending on the cultivar; flesh translucency; flesh bleeding from the pit; failure to ripen; and flavor loss. Susceptibility to IB varies greatly among stonefruit cultivars with a trend toward mid- to late-season cultivars being more susceptible than early-season types. Florida cultivars that are all early season and have the non-melting-flesh trait that tempers development of the abnormal flesh-related symptoms of IB tend to show less IB than the cultivars that are commonly grown elsewhere in the United States and in South America.
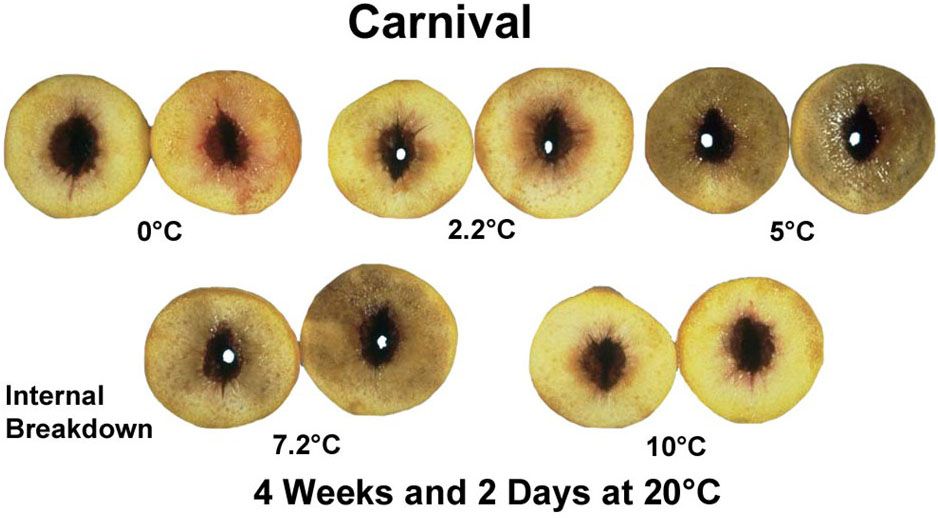
Credit: Don Edwards, U.C. Davis
The interrelation of several factors that influence IB make this a complicated disorder. Besides the variability in susceptibility among cultivars, there is also a fruit maturity component, with less mature fruit at harvest being more susceptible to IB than tree-ripe fruit. There is also a time-temperature interaction such that a long exposure to only slightly chilling temperatures can result in the same IB severity as a shorter exposure to a much lower temperature. Interestingly, the same low temperatures that cause IB also slow the rate at which the IB symptoms develop. Thus, IB is usually discovered during ripening after a cold storage and transportation period, that is, when the fruit are in the hands of the consumers. The first symptom of IB to develop is loss of or failure to develop normal ripe aroma, which means that slightly chilled fruit may look normal but never develop full flavor, leading to consumer complaints about “tasteless” stonefruit.
The worst postharvest temperature range for stonefruit in terms of IB, the so-called “killing zone,” is 36 °F to 46 °F (2.2 °C to 7.8 °C), which is where the temperature is sufficiently low to cause serious injury, but at the same time sufficiently high to allow IB symptoms to develop during storage and transport (Fig. 10-5). Traditionally, for domestic marketing of stonefruit, it has been recommended that the fruit should be rapidly cooled after harvest (within 8 hours) to near 32 °F (0 °C) and, counterintuitively, maintained at that low temperature throughout storage and transport. It has been found that with fruit kept near 32 °F (0 °C) within the time frame of 1 week or less that is required for domestic marketing, IB symptom development will be minimal.
Another strategy used to reduce IB that is widely practiced in California and Chile for stonefruit being shipped to relatively distant markets is “preconditioning,” which involves delaying the cooling of packed fruit for a couple of days so that their ripening can be initiated before they are exposed to low temperatures. This is equivalent to delaying harvest and cooling immediately as practiced for tree-ripe fruit. In either case, the more advanced fruit are less susceptible to IB than less mature fruit would be. However, tree-ripe melting-flesh cultivars are too soft to be run over standard packingline machinery without incurring significant bruising, so they are typically harvested earlier, with at least 8 lbs-force firmness, then packed, preconditioned, and cooled. In Florida, tree-ripe harvest of non-melting-flesh cultivars is a practical course of action that has the benefit of reducing the appearance of IB while providing full-flavored fruit to consumers.
Freezing Injury
Stonefruit flesh freezes in the range of about 27.5 °F to 30.5 °F (-2.5 °C to -1 °C) depending on the soluble solids content, which has an antifreeze-like effect in lowering the freezing point below that of pure water. When freezing occurs, the relatively dilute liquid between the cells freezes before the liquid cell contents, which creates a gradient favoring movement of liquid out of the cells into the intercellular space, where it in turn also freezes. If the low temperature exposure is brief, the ice will melt upon thawing, resulting in a watersoaked appearance, but the water will eventually be adsorbed back into the cells and the tissue can survive. With more severe or extended freezing conditions, the cells will eventually die due to dehydration, and, upon thawing, the affected tissue will remain watersoaked and become discolored. The appearance of freezing injury is characterized by its directionality: freezing and the freezing symptoms of watersoaked, discolored tissues, always proceeds from the outer to inner parts of the load, pallet, carton, and individual fruit.

Credit: Jeffrey K. Brecht, UF/IFAS
Heat Injury
Heat injury can manifest in several different ways, depending on the severity of the temperature and duration of exposure. Very high temperatures (around 125 °F [52 °C] or more) can cause scald-like surface injury and discoloration on stonefruit. Temperatures in the range of 86 °F to 104 °F (30 °C to 40 °C) in the orchard during delays between harvest and cooling can result in internal flesh browning of susceptible cultivars that develops during subsequent storage and shipping at proper temperatures. Holding stonefruit for extended periods (a day or more) at temperatures of 86 °F (30 °C) or higher can cause inhibition of softening and ripening or irregular softening and abnormal color changes.
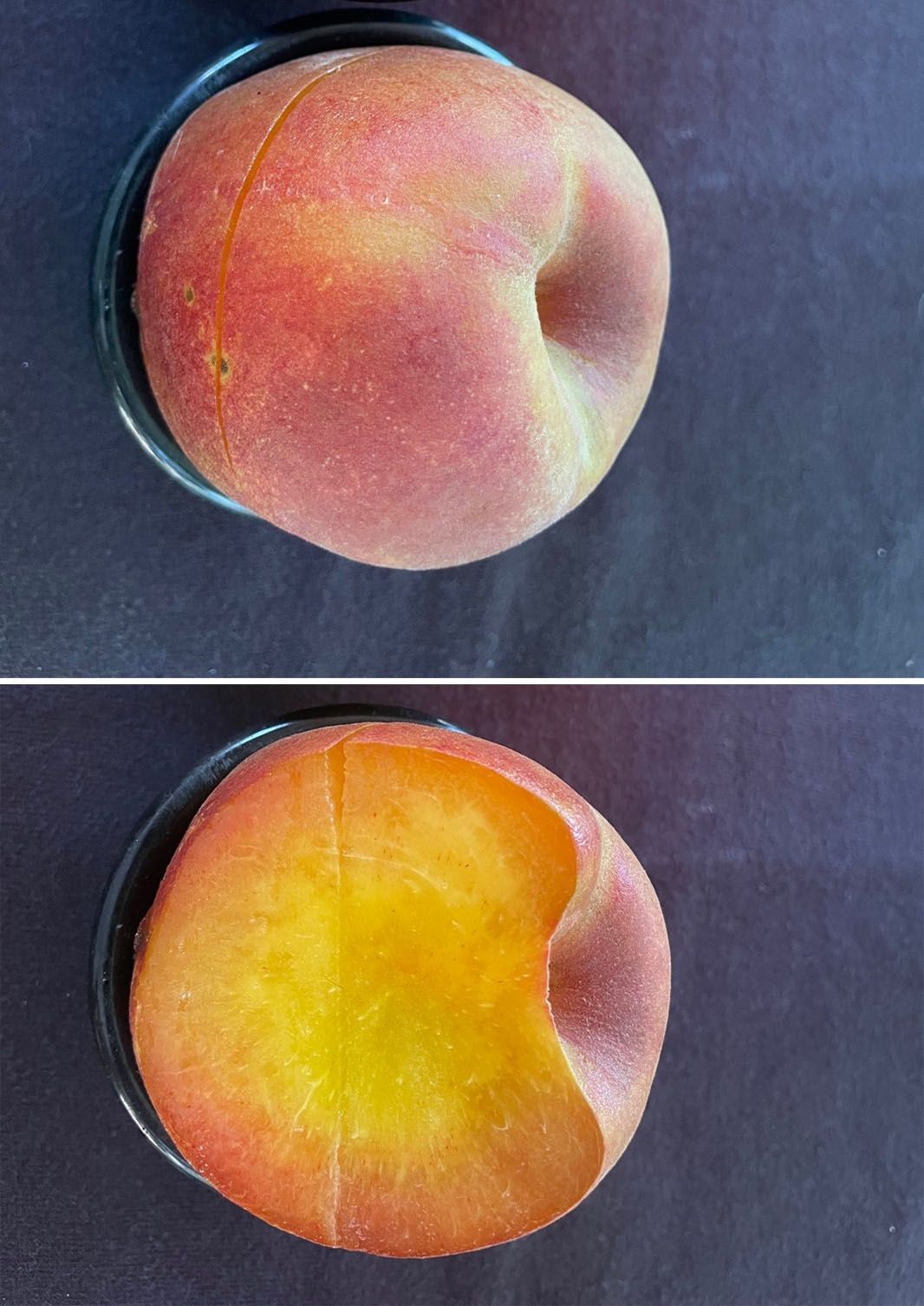
Credit: Jeffrey K. Brecht, UF/IFAS
Inking (Black Staining, Ink Spot, Peel Discoloration)
Inking is a cosmetic disorder that affects only the peel of stonefruit, but which makes the fruit unmarketable. The discoloration occurs in the fruit epidermis (outer peel tissue) and develops as brown, purplish, or black spots, stripes, or larger areas on the fruit surface, usually during the first 24 to 48 hours after harvest. Inking is related to surface abrasions or micro-injuries and exposure to water, which allows entry of heavy metals such as iron, copper, and aluminum into the peel tissue where they can react with phenolics and anthocyanins (red pigments) that are present in the peel to produce the inking symptoms. Abrasions and other injuries may occur during harvesting and bulk transport to the packinghouse or during postharvest handling, with inking having been associated with extensive exposure to grading rollers and brush beds before or after washing, rinsing, and hydrocooling. The heavy metals involved in inking may be present on the fruit surface at the time of harvest, or they may occur in the wash and rinse water or hydrocooler water. Iron has been found to cause the most severe inking symptoms.

Credit: Mercy A. Olmstead, UF/IFAS
To avoid inking, begin by avoiding foliar nutrient sprays within 15 days before harvest; fungicide sprays applied in accordance with label re-entry date requirements should not be a problem. Gentle handling during harvest and hauling reduces the injuries that allow heavy metal contamination of the peel tissue. Field bins should be kept clean and either made of plastic or fitted with plastic liners and have a foam-padded lid. Transport vehicles should have air suspension systems and be driven slowly, avoiding rough roads as much as possible to reduce vibration injury during transport from the orchard. For the same reason, the amount of time that the fruit spend on rollers or brush beds in the packinghouse should be minimized. If the water available for packinghouse operations contains heavy metals that are causing inking, it has been shown that maintaining the pH of chlorinated water at pH 6.5–7.0 reduces the concentration of free iron and significantly reduces inking. All water used in postharvest operations should be chlorinated as a matter of course for food safety reasons. Also, the metal chelator EDTA applied as a postharvest dip at 0.01 M (about 31 lbs/1,000 gal) pH 3.5–3.9 has been shown to reduce inking.
Senescent Flesh Browning
The flesh of fruit that have been stored for a long period may turn brown around the pit. This disorder is not due to chilling injury but is believed to be a symptom of advanced senescence or aging of the tissue. The membranes of senescent cells lose their ability to maintain cell contents separated in their proper compartments, leading to mixture of phenolic substrates and enzymes and oxidation of the phenolics, which turn brown.
Shriveling
Stonefruit lose water to their environment whenever a gradient exists between the aqueous interior of the fruit and the surrounding air. The symptoms of excessive water loss appear as loss of gloss on nectarines and plums followed by surface shriveling of peaches, nectarines, and plums. Shriveling usually becomes noticeable when the fruit have lost between 3% to 5% of their initial weight. Plums are more resistant to water loss than peaches and nectarines. Immature-harvested stonefruits are more susceptible to water loss and shriveling than mature fruit because the water-resistant cuticle on the fruit surface is less developed. When peaches are brushed or otherwise abraded and lose their “fuzz” (trichomes), the broken cells make the fruit more prone to lose water; application of wax coating can partially compensate for the broken trichomes, but brushed and waxed peaches still lose more water than un-brushed peaches.
To minimize water loss and shriveling, stonefruits should be cooled as quickly as possible to the desired storage and transport temperature and maintained in a high-humidity environment. A relative humidity (RH) of 85 to 95% is generally recommended for stonefruit as sufficient to minimize water loss. The exact humidity level that can be used depends on the temperature fluctuation that can be expected in the storage or transport environment such that the RH is as high as possible while avoiding condensation of liquid water. Condensation of water occurs when the air temperature during the cooling cycle of the refrigeration system goes below the dew point for that temperature and humidity combination. Coatings (waxes) that are available for use on stonefruits are water barriers and thus protect stonefruit from shriveling in lower humidity environments.

Credit: Mercy A. Olmstead, UF/IFAS
Additional Reading
Childers, N. F., and W. B. Sherman, editors. 1988. The Peach: World Cultivars to Marketing, 4th ed. Dr. Norman F. Childers Publications, Gainesville, Florida.
Crisosto, C. H., and D. Valero. 2008. “Harvesting and Postharvest Handling of Peaches for the Fresh Market.” In The Peach: Botany, Production and Uses, edited by D. Layne and D. Bassi, 575–596. CABI, Boca Raton, Florida. https://doi.org/10.1079/9781845933869.0575
Crisosto, C. H., and F. G. Mitchell. 2002. “Postharvest Handling Systems: Stone Fruits. I. Peach, Nectarine, and Plum.” In Postharvest Technology of Horticultural Crops, 3rd ed., edited by A. A. Kader, 345–350. Publ. 3311. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
LaRue, J. H., and R. S. Jonson (eds.). 1989. Peaches, Plums, and Nectarines. Growing and Handling for Fresh Market. Publ. 3331. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
Ogawa, J. M, E. I. Zehr, G. W. Bird, D. F. Ritchie, K. Uriu, and J. K. Uyemoto. 1995. “Compendium of Stone Fruit Diseases.” American Phytopathological Society, St. Paul, Minnesota.
Rushing, J. W., and K. C. Taylor. 2002. “Post-Harvest Management: Peach Skin Discoloration and Water Quality Management.” Southeastern Peach Growers’ Handbook. University of Georgia College of Agric. & Environ. Sci., Athens, GA. p. 121–126.
10. Distribution, Marketing, and Consumer Handling of Florida Stonefruits
Jeffrey K. Brecht and John Van Sickle
Most stonefruits are sold by packer-shippers to supermarket chains or food service companies and shipped directly from the packinghouse to the buyer’s distribution center (DC). Smaller amounts of stonefruits are sold to terminal market buyers, brokers, and independent wholesalers, who in turn sell the fruit to retail stores, restaurants, and institutions. A few stonefruits are sold directly to consumers at farmers markets and roadside stands. As stated in the chapter on stonefruit harvest operations, the harvest maturity of the fruit, grading and packing systems, choice of packaging, temperature management, and other aspects of handling need to be appropriate for the intended market.
Inspections at Origin
Stonefruit packer-shippers should have a quality control (QC) program in place to monitor all parts of the operation to ensure that the packed fruit meet the desired quality standards for customers and labels. In production areas that are subject to a marketing order, stonefruit may be inspected at the packinghouse by state or federal inspectors to ensure that the fruit being packed meet grade standard requirements related to such things as maturity, size and uniformity, freedom from defects, and package weight or dimensions. It is becoming more common for large buyers to station personnel in major production areas to inspect the quality of fruit being packed before confirming purchase and loading. Buying agents and brokers can serve a similar function for smaller companies buying stonefruit.
Holding Sample Lots of Fruit at the Packinghouse for Quality Control
For QC, it is recommended that a representative sample (at least 25 randomly selected fruit or one carton of each fruit size) from each lot that passes through the packinghouse be retained in the storage room while the rest of the lot is being shipped and has been delivered to the buyer. When the packer/shipper learns that the buyer has received the shipment, personnel should transfer the QC fruit sample to an air-conditioned room, such as an office, at 75 °F to 77 °F (24 °C to 25 °C) to complete ripening. This procedure allows the packer/shipper to compare the quality of the fruit under ideal storage and ripening conditions to the reported quality of the shipped fruit. This can provide evidence as to whether discrepancies that might be noticed by receivers are due to the different conditions to which the fruit were exposed during distribution as opposed to problems with initial fruit condition.

Credit: Jeffrey K. Brecht, UF/IFAS
Unloading and Holding on the Dock at the Receiver Facility
Stonefruit should be unloaded directly from shipping containers and trailers onto a refrigerated receiving dock at the receiver in order to maintain the integrity of the cold chain. Receivers should retrieve every temperature recorder from the shipment, document the specific location of each recorder in the load, retain and copy the entire label and strip chart or recorder download, judiciously review the temperature records, and then send the recorders to the manufacturer for post-trip calibration if temperature management problems are suspected. The transportation unit refrigeration should be turned off while the fruit are being unloaded. Running the refrigeration while unloading cargo can cause the transfer of unwanted hot or cold ambient air and exhaust fumes into the cargo space.
Holding time on the receiving dock should be limited to that required for identifying and recording the load and retrieving temperature recorders. Pallets of stonefruit should be moved from the container or trailer directly to the receiver’s cold-storage area; pallets should not be held for any significant amount of time on the receiving dock. There should be a workspace available immediately inside the cold-storage area to hold fruit for inspection prior to stowing the pallets in the cold room and/or on pallet racks.

Credit: Jeffrey K. Brecht, UF/IFAS
Inspection at the Receiver Facility
The QC inspection that is performed upon arrival at the receiver’s facility determines whether a shipment will be accepted or rejected, as well as its potential utilization. This is an extremely important QC point that has a great effect on the company's bottom line. It should never be rushed or cursory. Assign no more than one or two people to conduct QC inspections for uniform and repeatable results. If additional inspectors are required due to the volume of products being inspected, these inspectors must be adequately trained and certified to assure uniform and repeatable results.
The following procedure is recommended for evaluating the quality of a load of stonefruit. Take single-carton samples in a standard pattern from the front, middle, and rear (door) areas of the load, sampling the top, center, and bottom of the pallets on both the left and right at each of those three areas, for a total of 18 sample cartons. Immediately measure the flesh temperature as the pallets are being unloaded and the samples are being collected. Take flesh temperature readings from three basic areas within the trailer or container (i.e., the front, the middle, and the rear door areas). Ideally, the temperature at the upper left, upper right, center, lower left, and lower right should be measured in all three areas during an inspection (for a total of 15 readings). The flesh temperature of stonefruit on arrival should be between 32 °F and 35 °F (0 °C and 1.7 °C).
Document the visual appearance of the fruit, cartons, and pallets with a standard battery of digital photographs. Evaluate the fruit for: 1) overall condition and ripeness; 2) flesh color, firmness, and soluble solids (°Brix); and 3) incidence and severity of defects, damage, disorders, and decay, both externally and internally.

Credit: Jeffrey K. Brecht, UF/IFAS
Sorting at the Receiver Facility
If the receiver is an independent wholesaler or a repacker, fruit may be sorted to meet customers’ specifications at their facility; however, it is best if this type of sorting is done mainly at the packinghouse in the growing area. If sorting is required before stonefruits are delivered to customers, ensure that the QC personnel know how to recognize the most important external defects to take into account when stonefruits are being sorted. Fruit that does not make the grade may be suitable for another market outlet depending on the condition. A simple sorting table can be used to sort the fruit for visual appearance, damage, decay, excess softening, or internal breakdown to meet grade standards or customer specifications. The tables should be at a comfortable height for workers with adequate lighting directed onto the sorting table and not into the sorters' eyes. Belts to transport and rotate the fruit assist sorting speed and accuracy. Fruit must be handled gently by workers and equipment to prevent impact injury during sorting and repacking. Fruit should be returned to the same cartons after sorting to maintain traceback capability. Fruit should be packed and re-palletized gently.

Credit: Pavlos Tsouvaltzis, UF/IFAS
Storage at the Receiver Facility
Ideally, stonefruit should not be stored at a DC for longer than the time required to fill orders for shipping to retail stores or other customers. However, marketing considerations may dictate that larger, perhaps several-day supplies of fruit must be accommodated from time to time. Pallets of stonefruit should be stored on racks in a cold room set to a temperature between 32 °F and 35 °F (0 °C and 1.7 °C); the only reason to hold stonefruit at higher temperatures would be when the fruit are held at 20 °C to 22 °C to promote ripening. However, unlike some other fruits like bananas and avocados, stonefruit do not usually require, and do not receive, special ripening treatments. Temperatures in the so-called “killing zone” of 36 °F to 46 °F (2.2 °C to 7.8 °C) should be strictly avoided because chilling damage to stonefruits is most severe in that temperature range. Maintain the relative humidity in the area where stonefruits are being held at 90% to 95%. Scrub ethylene gas from the cold room air with an absorbent or provide one full fresh air exchange each day.
Staging for Loading at the Receiver Facility
Most large DCs fill orders for their retail stores 6 days per week by assembling pallets of mixed products. These pallets are typically assembled and staged on the trailer loading dock. The dock staging area should be protected from the sun and refrigerated where possible. If the dock area cannot be properly refrigerated, stage loads in the cold-storage area, instead. Load pallets directly from the cold room into the trailer to avoid warming. Air temperatures of 32 °F to 35 °F (0 °C to 1.7 °C) in the staging area are ideal.

Credit: Pavlos Tsouvaltzis, UF/IFAS
Transport to Retail Stores
Prior to loading of trailers for deliveries to retail stores, trailers should be inspected to ensure cleanliness. Clean and sanitize the trailers if necessary before loading any product. Avoid hasty loading operations at the receiver facility because they can result in damage to pallets that creates unstable conditions for the stonefruit cartons, leading to potential for further damage. Use air bags or other bracing materials between pallets and between pallets and trailer walls to protect pallets from movement (leaning) during deliveries to retail stores.
Stonefruits are typically delivered to retail stores in truck trailers containing a wide variety of produce and other food items. Develop a delivery plan that ensures stonefruits are loaded into trailers in the most conducive location for maintaining their optimum temperature when several commodities are being delivered in mixed loads. Supervise the trailer loading operation. The carrying temperature chosen is always a compromise among the products being carried and may not be best for stonefruits. The highest typical temperature used for retail store deliveries is 59 °F (15 °C). Exposure to such a temperature for relatively short typical delivery times of up to 6 hours should not be too detrimental to stonefruit quality. However, care should be taken to minimize exposure of stonefruits to extreme outside temperatures during loading and unloading that could result in freezing or heat injury.
Trailers used for deliveries should be periodically inspected to ensure that temperatures are well-maintained. Repairs should be made to damaged doors and sidewalls if the damage allows air leaks. Refrigeration drain pans should be maintained in good order to prevent dripping condensation from causing water damage to fiberboard cartons.
Unloading at Stores and Holding on Docks at Stores
Upon arrival at the retail store, products are unloaded according to the requirements of the store purchase order. Depending on the arrival time and the availability of personnel to receive the products, the receiving process can vary widely from one retail store to another. Trailers should remain closed until the store personnel responsible for the reception process arrive. Otherwise, the temperature inside the trailer can warm (or chill) significantly due to infiltration of outside air.

Credit: Jeffrey K. Brecht, UF/IFAS
Every effort should be made to minimize the time that trailer doors are open at the retail store. Trucks deliver products to more than one retail store, and the drop-off time at each store cumulatively exposes the trailer contents to damaging outside temperature extremes (hot or cold). Train retail store personnel in produce temperature sensitivity, including that of stonefruits. Show them how stonefruits can suffer due to exposure to temperature extremes.
Designate retail store personnel to help in product unloading to minimize product exposure to damaging outside temperatures. Perform a QC inspection at the retail store upon delivery and provide prompt feedback to the DC regarding the results of the inspection. Use the information to make improvements to handling practices at the DC and during delivery.
Storage in Walk-In Coolers at Stores
The retail store may not receive shipments of stonefruits every day; rather, an inventory of stonefruits may be kept in a walk-in produce cooler for 2, 3, or 4 days. The temperature of these storage rooms is typically maintained at 41 °F (5 °C), which is damaging to stonefruits. A few, well-designed stores have separate storage rooms at 32 °F to 36 °F (0 °C to 2.2 °C) and 45 °F to 50 °F (7.2 °C to 10 °C) for produce with different temperature requirements. Tropical products and fruit that are judged to require extra ripening time may be held in an air-conditioned preparation room. The temperatures found in many retail store walk-in coolers during store operating hours are often higher than the set point because of numerous entries and exits by personnel. Many retail stores protect their walk-in produce coolers by using strip curtains over doorways, but this method is not effective if the curtains become damaged and allow warm air to seep into the cooler. Occasionally, poorly trained store personnel deliberately cut the strips to ease entry/exit. This practice is ruinous to temperature-sensitive produce like stonefruits. Further, wall thermometers in these coolers are often poorly monitored and maintained, or they are placed improperly, leading to inaccurate temperature readings.

Credit: Megan Kinsman, UF/IFAS
The produce manager of a retail store should regularly inspect the back room and cooler area. The following points should be emphasized:
- Walk-in cooler doors should be kept open only for the time necessary to enter or exit; strip curtains should be used on the walk-in cooler doors and must be kept in good repair.
- Calibrated thermometers should be placed in the back room and cooler and positioned away from doors or radiant heat sources (motors or lights) in order to indicate representative temperatures.
- Placement of produce in the cooler room should take into account product temperature requirements.
Since cooler space with optimum conditions for stonefruits may not be available, stonefruits should be ordered frequently enough that they are held at back-room temperatures for no more than a day or two. It is best to place stonefruits on display as soon as they are delivered to the retail store. Order them more frequently to avoid storing fruit at the store.
Stocking and Display Preparation and Rotation
Product storage at the retail store in the back room or in a walk-in cooler is the last step in the cool chain for stonefruits that extends all the way back to the orchard at the time of harvest. Maintaining good temperature control right up to when the fruit are displayed for sale by retailers has a positive effect on the shelf life of stonefruits. Minimizing shrink, mechanical injuries, and water loss will also help retail stores realize maximum sales.
It is desirable to display stonefruits that are ready to eat. An artful display of ripe fruit creates a major visual impact and showcases the fruit’s best organoleptic characteristics (which means qualities that appeal to the senses—the heavenly smell of a ripe peach, for instance). Stonefruits should be displayed in an open area in the retail store, not in a refrigerated product merchandiser. This allows the fruit aroma to develop and attract shoppers. Stonefruits should not be displayed as a large mountain or pyramid of fruit because ripe stonefruits are susceptible to compression bruising. Ripe fruit can bruise from even a very small amount of pressure; even the weight of one fruit on top of another can bruise the bottom fruit. Consider having two different displays or display sections for each type of stonefruit. Locate fruit that are less ripe and will be ready to eat in a day or two on one side and riper fruit that are ready to eat right away on the other side.

Credit: Jeffrey K. Brecht, UF/IFAS
A program of regular cleaning and sanitation of the produce area is key to keeping the displays pleasing and attractive. Rotating the stonefruit displays frequently by moving older fruit from the bottom to the top or center of the display is an important management practice that encourages greater overall sales. Inspect the display several times each day and immediately remove overripe, shriveled, leaking, injured, and damaged or decayed fruit. If possible, when adding new fruit to those already displayed, it is a good idea to keep the fruit separated by ripeness stage so that shoppers can more easily locate fruit at the ripeness stage they prefer, reducing the amount of handling that individual fruit receive.
Recordkeeping
Keeping records is an important part of a QC program at any stage of stonefruit handling. If whatever is done is not recorded, it is as if it was never done when the time comes to show an inspector that best practices were followed. Each operation should assign an employee to be responsible for the QC program. The QC manager should bring together the most knowledgeable people in the operation to prepare a list of all the operations and procedures conducted in the facility. The QC manager should then develop a form for recording that all of those operations and procedures are being done—and done correctly and on a regular basis.
Consumer Handling
When stonefruits are brought home from the store, their taste and aroma is typically best when the fruit are kept at ambient room temperature. Stonefruits that are not ready to eat when purchased require further ripening in the consumer’s home, which can be best accomplished by holding them in a paper bag on the counter, out of the sun to avoid excessive heat. Perforated plastic bags and special fruit-ripening bowls are even better because moisture loss and thus shriveling is reduced. Placing an apple or an avocado in the bag or bowl with the stonefruit will speed up ripening, because those fruits produce large amounts of ethylene gas, which promotes stonefruit ripening.
The temperature of typical home refrigerators is about 40 °F (4.4 °C), which is squarely within the stonefruit killing zone for internal breakdown. However, the negative effects of low temperatures on stonefruits decline as the fruit ripen, so stonefruits that are purchased ripe, or that have ripened adequately on the kitchen counter, can be safely held for a day or two in the refrigerator without undo damage to their taste and texture.
Additional Reading
Collins, R. 2014. “Value Chain Management and Postharvest Handling.” In Postharvest Handling: A Systems Approach, edited by W. J. Florkowski, R. L. Shewfelt, B. Brueckner, and S. E. Prussia, 123–145. Academic, San Diego, California. https://doi.org/10.1016/B978-0-12-408137-6.00006-5
Crisosto, C. H., and F. G. Mitchell. 2002. “Postharvest Handling Systems: Stone Fruits. I. Peach, Nectarine and Plum.” In Postharvest Technology of Horticultural Crops, edited by A. A. Kader, 345–350. Pub. 3311. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
Crisosto, C. H., and D. Valero. 2008. “Harvesting and Postharvest Handling of Peaches for the Fresh Market.” In The Peach: Botany, Production and Uses, edited by Desmond Layne and Daniele Bassi, 575–596.Wallingford, Oxfordshire, GBR: CABI Publishing., U.K. https://doi.org/10.1079/9781845933869.0575
Mitchell, F. G. 1989. “Distribution.” In Peaches, Plums, and Nectarines. Growing and Handling for Fresh Market, edited by J. H. LaRue and R. S. Johnson, 223–229. Publ. 3331. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.
Mitchell, F. G., and A. A. Kader. 1989. “Field Handling and Packing.” In Peaches, Plums, and Nectarines. Growing and Handling for Fresh Market, edited by J. H. LaRue and R. S. Johnson, 197–208. Publ. 3331. Cooperative Extension, University of California Division of Agriculture and Natural Resources, Oakland, California.