Achieving optimal nutrient use efficiency and growth of specialty crops requires careful management of the irrigation water’s pH, especially when the water quality exhibits excessive alkalinity. In Florida, where approximately 90% of the water supply comes from the Floridan Aquifer, the water typically has a pH of approximately 8.10 due to its limestone and dolostone composition. This high pH can lead to issues such as clogging or blocking of fertigation system components like emitters due to carbonate, sulfate, or phosphate deposits.
Given these challenges, adjusting the pH of irrigation water is often necessary to align with crop preferences and create optimal growing conditions. Raising the pH of irrigation water is usually not advisable; instead, lowering the pH is often essential. This publication provides practical recommendations for adjusting irrigation water pH to address these issues effectively. The target audiences of this guide are farm and nursery managers, growers, crop advisors, Future Farmers of America (FFA), Extension agents, researchers, students, and others who are interested in crop production.
What is pH?
The acidity or alkalinity of water is measured using pH, a scale derived from the negative logarithm (-log10 or simply -lg) of the hydrogen ion (H+) concentration with a base of 10. The pH level signifies the "potential concentration of hydrogen ions" in a solution. It gauges the solution's acidity or alkalinity on a scale ranging from 0 to 14, with 7 representing neutrality (as depicted in Figure 1) (Liu and Hanlon 2024). Solutions registering a pH below 7 are categorized as acidic, while those above 7 are deemed basic or alkaline. In agriculture, pH plays a pivotal role in influencing nutrient availability in soil, enzymatic activity in plants, and various other factors vital to plant growth and development. Thus, monitoring and managing pH levels are critical aspects of farming practices.
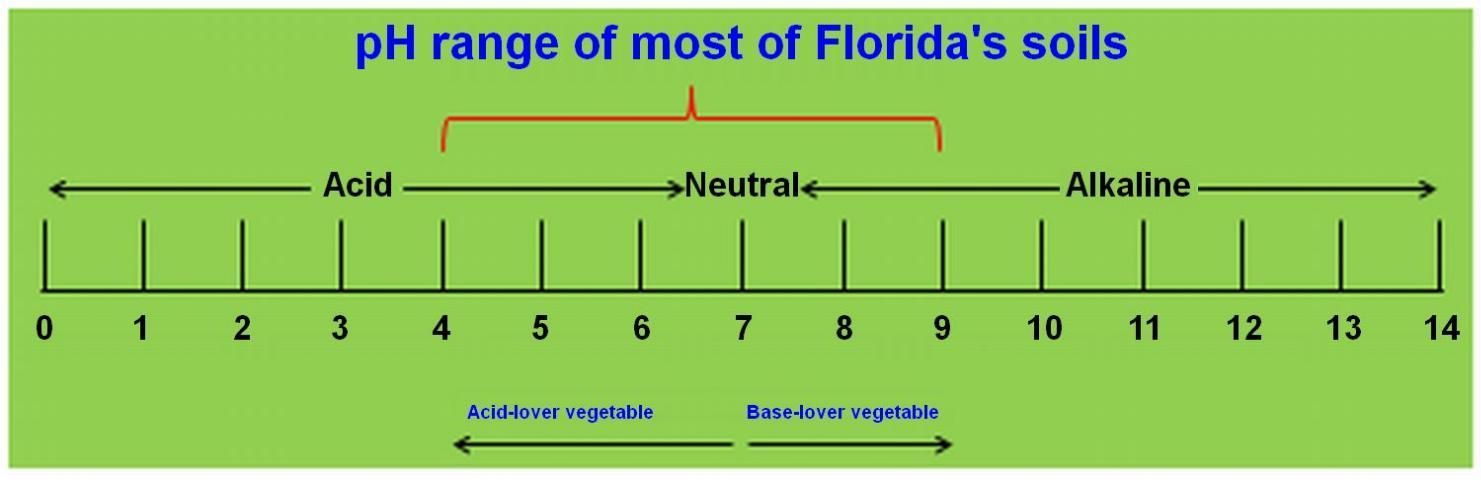
Credit: Guodong Liu, UF/IFAS (Liu and Hanlon 2024)
Why does the pH of irrigation water need to be adjusted?
There are two primary reasons for adjusting irrigation water’s pH: water quality and crop preferences.
- Water Quality: Around 90% of the irrigation water is sourced through the Floridan Aquifer from wells. This water contains approximately 350 ppm bicarbonate (HCO3-), primarily as calcium or magnesium bicarbonate (Sprinkle 1989). Consequently, the pH of well water typically measures around 8.10. In April 2024, we conducted measurements of well water pH at the UF/IFAS Plant Science Research and Education Unit in Citra, Florida, and found the water pH ranged from 7.94 to 7.96. Despite this minor deviation, elevated pH levels cause many micronutrients to oxidize, rendering them unavailable to crops. For instance, most vegetable crops rely on ferrous iron (Fe²⁺), which begins to oxidize into ferric iron (Fe³⁺) when the pH exceeds 5.3 (Liu et al. 2024). Therefore, it is imperative to lower the pH by acidifying the irrigation water with an appropriate acid solution to mitigate these issues.
- Crop Preference: Each crop exhibits its unique preference regarding soil pH levels for optimal growth and development. While most crops thrive within a pH range of 5.5 to 6.5, some crops demonstrate a preference for lower pH conditions. For instance, blueberries flourish in acidic environments, with an ideal pH range of 4.5 to 5.5 (Liu and Hanlon 2024). Similarly, raspberry, mustard greens, and turnips are other examples of a crop that thrives in acidic soil, with an ideal pH range of 4.2 to 5.5 or 5.5 to 6.8 (Smith et al. 2007). A common agricultural practice is the cultivation of cranberries in peat bags or marshy areas where the soil naturally maintains an acidic pH.
Essential Tools to Adjust the pH of Irrigation Water
- A pH meter serves as a crucial tool for measuring pH levels to determine the necessary amount of acid required for specific irrigation or fertigation events. Over 10 styles of pH meters are available on the market, ranging from high-end models with prices around $2,400 per meter to more economical options costing less than $10 per meter. Given the practical demands of fieldwork, a digital pH meter with high accuracy, measuring pH to 0.01 increments, is well-suited for acid quantification purposes. Figure 2 depicts the pH meter utilized in our field trials conducted at the UF/IFAS Plant Science Research and Education Unit in Citra, Florida.

Credit: Guodong Liu, UF/IFAS
2. The pH Buffer solution is typically used for pH meter calibration in research settings and is relatively expensive. Powder packets are more cost-effective but require the user to prepare the standard pH solution. Powder packets come in three separate bags, each designated for a different pH standard solution. These bags are distinguished by their respective colors: red for pH 4.00 or 4.01, green for pH 6.86 or 7.00, and blue for pH 9.18 or 10.01 (see Figures 3 and 4). Both standard pH buffer solutions from the market and those prepared with the powder packets work effectively, but the latter may be more convenient for growers to use in the field. You can prepare the standard solutions either indoors or in the field with purified water according to the instructions. All buffer solutions from both sources can be used for up to two years if stored properly in sealed brown bottles at room temperature. Fertigate your crops whenever is convenient to you, but it is better to adjust your pH before you run fertigation. Additionally, please avoid scheduling your fertigation events on days with heavy rain, as this can lead to nutrient leaching problems.

Credit: Guodong Liu, UF/IFAS

Credit: Guodong Liu, UF/IFAS
3. Acid solution is the product injected into the drip irrigation line and can be prepared using various sources, such as 10% or 40% sulfuric acid (see Figure 5) or phosphoric acid (see Figure 6), especially when liquid phosphorus fertilizer (e.g., 0-54-0) is applied through the drip irrigation system. Both sulfuric acid and phosphoric acid are commercially available. It is easy to find highly concentrated sulfuric acid (e.g., 93%, 98%) on the market. However, it is crucial to emphasize that we strongly discourage the use of such high concentrations due to safety concerns and corrosivity.

Credit: Guodong Liu, UF/IFAS

Credit: Guodong Liu, UF/IFAS
4. A disposable plastic liquid dropper is employed for calibrating the pH of irrigation water when utilizing a 5-gallon bucket.

Credit: Guodong Liu, UF/IFAS
5. A 5-gallon bucket is used to determine the quantity of acid solution required for each fertigation event to achieve the targeted pH of fertigation water.
Adjusting the pH of Irrigation Water
There are six steps to achieve suitable pH for effective fertigation in vegetable and fruit production. As an example, we will calculate optimal pH for snap beans from our field trial site at the UF/IFAS Plant Science Research and Education Unit.
Step 1: Fill the 5-gallon bucket with the source water.
Step 2: Measure the pH of the water. We measured the source water pH in late April, obtaining a reading of pH 7.96 (Figure 8). For this trial, we aimed for a target pH of approximately 4.00. You can find the optimal pH for major vegetable and fruit crops from these EDIS articles: https://edis.ifas.ufl.edu/publication/HS1207 and https://edis.ifas.ufl.edu/publication/HS1234.
Step 3: Using the disposable plastic liquid dropper (Figure 9), carefully add drops of 10% sulfuric acid to the source water. Stir thoroughly to mix, and then measure the pH again. Add the acid slowly and cautiously, as the pH can change suddenly. Record the total amount of acid added as you proceed.

Credit: Md Jahidul Islam Shohag and Guodong Liu, UF/IFAS

Credit: Md Jahidul Islam Shohag and Tian Shufang, UF/IFAS
Step 4: Once the pH reaches the target level (e.g., pH 4.05; see Figure 10) after adding the acid, calculate the total volume of acid added. In the example provided, we added 10 mL of 10% sulfuric acid solution to 5 gallons of water. This equates to 2 mL per gallon.

Credit: Md Jahidul Islam Shohag and Guodong Liu, UF/IFAS
Step 5: Determine the acid requirement for a specific fertigation event based on the acreage you will be fertigating. For example, if you need to acidify 5 acres of snap bean using the previously prepared water and acid, with a total irrigation water volume of 1000 gallons for the fertigation event, you will require 2000 mL of 10% sulfuric acid or 0.53 gallons of 10% sulfuric acid. If you use 40% sulfuric acid, 17 ounces of the acid is needed. Then, inject this amount of acid into your fertigation system using an injector or a dosatron.
Step 6: Check the pH of the water from your drip line. Place a 250 mL cup into the soil under the drip line to collect the fertigation water, then measure the pH. In our example, we recorded a pH of 4.50 (Figure 11).

Credit: Md Jahidul Islam Shohag, UF/IFAS
Special Note
To lower soil pH effectively, it is crucial to ensure that the pH of irrigation water is lower than the desired soil pH. The precise pH of the irrigation water depends on both the current soil pH and the target pH level. For instance, if the soil pH is too high and needs to be reduced, the pH of the irrigation water should be lower than the current soil pH. It is important to reduce the soil's pH gradually over time. Along with adjustments to the pH of irrigation water, regular monitoring of soil pH is essential to achieve the optimal soil acidity level for ideal plant growth.
References
Liu, G. D., and E. Hanlon. 2024. “Soil pH Range for Optimum Commercial Vegetable Production.” HS1207. University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/HS1207
Liu G. D., R. Mylavarapu, E. Hanlon, and W. C. Lee. 2024. “Soil pH Management for Optimum Commercial Fruit Production in Florida.” HS1234. University of Florida Institute of Food and Agricultural Sciences. https://edis.ifas.ufl.edu/publication/HS1234
Smith, B. R., D. L. Mahr, P. S. McManus, and T. R. Roper. 2007. Growing Raspberries in Wisconsin. A1610. SR-04-2007-(R11/01)-2M. University of Wisconsin-Extension, Cooperative Extension. https://barron.extension.wisc.edu/files/2023/02/Growing-Raspberries-in-Wisconsin.pdf
Sprinkle, C. L. 1989. Geochemistry of the Floridan Aquifer System in Florida and in Parts of Georgia, South Carolina, and Alabama. Regional Aquifer-System Analysis. U.S. Geological Survey Professional Paper 1403-1. https://doi.org/10.3133/pp1403I
Further Reading
Atucha, A. 2020. "Adjusting Soil pH for Cranberry Production." Wisconsin Fruit News, April 24. https://fruit.wisc.edu/2020/04/24/adjusting-soil-ph-for-cranberry-production/#:~:text=The%20ideal%20pH%20for%20cranberries,the%20soil%20(Figure%201)