The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
Introduction
Anisakid nematodes are parasites of animal intestines and cause the disease anisakiasis in humans, usually involving only mild symptoms of abdominal pain, malnutrition, and vomiting. However, the larval form of these nematodes does not persist in humans, and infections usually resolve by themselves. Human infection from anisakid nematodes comes inadvertently through consumption of raw fish, as the causal agent, Pseudoterranova decipiens, infects marine life and generally takes up residence in the stomach of gray seals (Halichoerus grypus) or other large seals. However, the host distribution of Pseudoterranova decipiens is extraordinarily broad and many other marine animals can serve as hosts (e.g., crustaceans, other large invertebrates, and many fish species). Pseudoterranova decipiens is considered a species complex containing other named species with very similar characteristics (Marcogliese 2001; CDC 2019).
Synonymy
Reported synonyms for Pseudoterranova decipiens (Krabbe, 1878) Gibson, 1983 are Ascaris decipiens (Krabbe, 1878); Phocanema decipiens (Krabbe, 1878) Myers, 1959; Porrocaecum decipiens (Krabbe, 1878) Baylis, 1920; and Terranova decipiens (Krabbe, 1878) Mozgovoi, 1953 (Bezerra et al 2020).
Distribution
Pseudoterranova decipiens and related nematodes are widely distributed, occurring nearly anywhere where adequate populations of host marine animals can be found, such as cold-water coastal regions. This includes the eastern coast of Canada, the northeastern United States, Iceland, Greenland, Philippines, Japan, Northern European shores, and parts of South America (Mercado et al. 2001, McClelland 2002, Hernandez-Orts et al. 2013, Skrzypczak et al. 2014, Al Quraishy et al. 2019).
Description
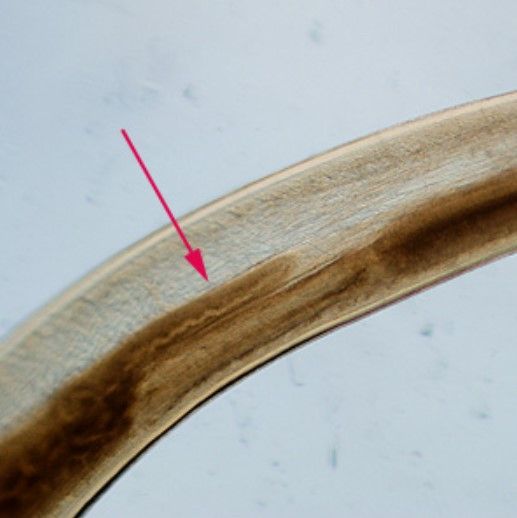
Pseudoterranova decipiens is a relatively small nematode (10 to 12 mm (~3/8 to ½ in)) with a prominently needle-shaped, piercing stylet; three lips are visible (Figure 1). The intestinal cecum (the pouch that connects small and large intestines) is visible and assists with diagnosis under light microscopy (Figure 2). In cross-section, Pseudoterranova decipiens displays a prominent shaped set of hypodermal chords (Figure 3). Laboratory and medical diagnosis of infection is done via gastroscopic examination in which worms are removed from the patient for identification. Also, worms that may be coughed up by the patient can be preserved for diagnostic examination (CDC 2019).

Credit: Centers for Disease Control and Prevention, https://www.cdc.gov/dpdx/anisakiasis/index.html
Credit: Centers for Disease Control and Prevention, https://www.cdc.gov/dpdx/anisakiasis/index.html
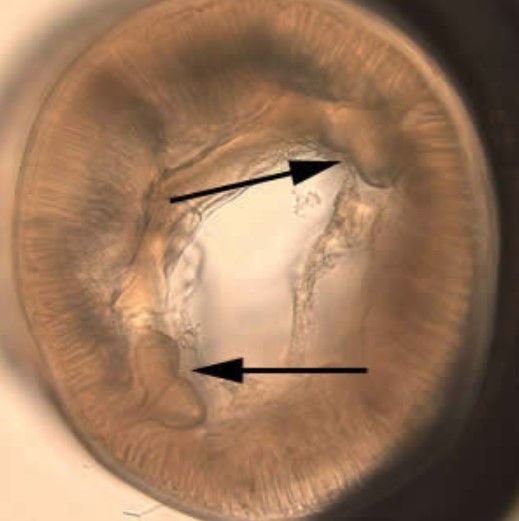
Credit: Centers for Disease Control and Prevention, https://www.cdc.gov/dpdx/anisakiasis/index.html
Life Cycle and Biology
Pseudoterranova decipiens are gonochoristic (distinct male and female individuals), with both sexes present in the host gut (Levsen et al. 2008). Eggs produced within the host are voided through excreta into the water. The first-stage and second-stage larva (L1 and L2) may form in the eggs and then embryonate (develop into an embryo) to form free-swimming third-stage larvae (L3); alternatively, eggs may be ingested by small crustaceans such as amphipods and mysids, which are then eaten by small fish where the larvae develop (McClelland 2002). Prey fish then consume L3 larvae or the small intermediate host fish through predation, and these larvae then migrate within the fish to muscle tissues where they will pass to other prey fish species of larger size until they finally reach the gut of their definitive mammal host where final molts occur in the gut prior to reproduction. However, should infected fish be caught and served to humans, L3 larvae can cause demonstrable harm in the accidental host (CDC 2019). Note the transfer to the accidental host through ingestion of raw or undercooked fish, notably cod and other common sushi/sashimi fishes, highlighting the importance of appropriate management strategies (Figure 4).
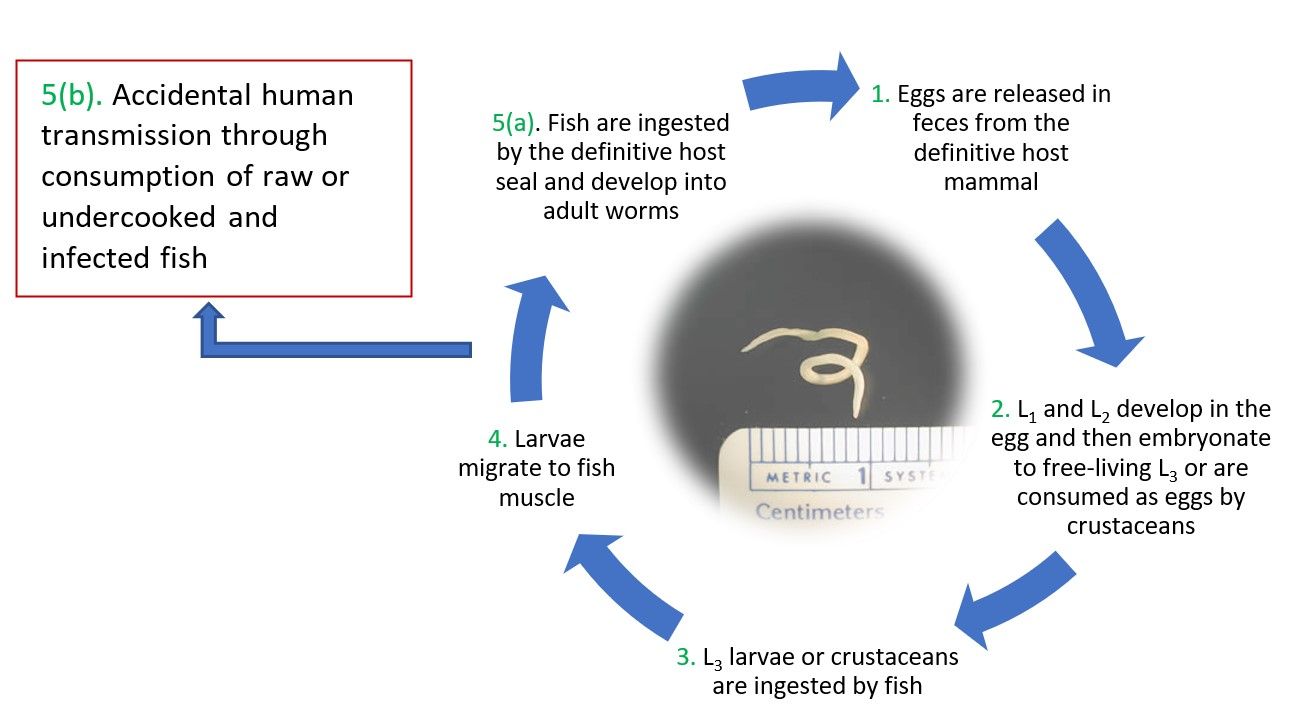
Credit: Centers for Disease Control and Prevention, https://www.cdc.gov/dpdx/anisakiasis/index.html
Hosts
Pseudoterranova decipiens generally takes up residence in the stomach of gray seals or other large marine pinnipeds (e.g., common seals, Phoca vitulina), as the preferred definitive host. However, there are several known intermediate and paratenic hosts (hosts that provide a home until a preferred host is found), including over 40 invertebrate species, and over 60 species of fish (Marcogliese 2001).
Medical Importance
Parasitic infection with members of the Anisakidae (including Pseudoterranova decipiens and related species) is termed anisakiasis, though this can refer to infection from any member of the family. Regardless of the classification of the disease, the symptoms are quite similar. Because the nematode is ingested, and it is naturally a gut parasite, most described symptoms are gastrointestinal, and most cases are mild and self-limiting. This pain has been described as “prickling epigastric pain” with rapid onset within two days of consuming raw fish (Yu et al. 2001). However, some clinical reports describe a variety of symptoms if the nematode migrates out of the gut, including coughing, pruritis (itching) of the nose and anus, and pharyngeal (throat) pain (Mercado et al. 2001). Additionally, there is a reported case of a male patient with suspected hepatic metastatic disease (liver cancer) who was, upon laparoscopic examination, found to have anisakiasis, resulting in a 20 mm (¾ in) liver mass (Murata et al. 2018). Finally, Pseudoterranova decipiens proteins can cause a severe and medically dangerous allergenic response in mice and is suspected to cause similar problems in humans with repeated exposure to this parasite (Ludovisi et al. 2017).
Economic Importance
Because Pseudoterranova decipiens is found in common fishes eaten by humans, there is the possibility of infestations causing a heavy economic toll. In fact, though recent estimates are not available, the annual cost to fish processors was estimated in 1986 as $26.6 million annually (Malouf 1986) and as $50 million annually in 1991 (Aryee and Poehlman 1991). These estimates are exclusive of the potential economic costs of healthcare to patients accidentally infected by these parasites and are generally attributed to the costs associated with handling, screening, inspecting, and removing nematode parasites from fish.
Management
As with any sound management strategy, simple solutions pay large dividends. Management through detection and removal is costly. However, though the sight of worms may be unpalatable, Pseudoterranova decipiens can be rendered harmless to human by either freezing fish meat before using it for sushi/sashimi preparations, or by cooking the flesh of the fish prior to consumption, as either temperature extreme kills the parasite (Goater et al. 2014). Medical management remains costly for those infected, as gastroscopic examinations can be expensive, removal of nematodes notwithstanding. Management of this species is likely to continue to be a priority for fisheries, as a recent meta-analysis suggests that populations of this nematode complex have increased 283-fold over 37 years (Fiorenza 2020). This could be the result of changes in host populations as well as long-term climate change.
Selected References
Al Quraishy S, Abdel-Gaber R, Dkhil MAM. (2019). First record of Pseudoterranova decipiens (Nematoda, Anisakida) infecting the Red spot emperor Lethrinus lentjan in the Red Sea. Brazilian Journal of Veterinary Parasitology 28: 625-631. https://doi.org/10.1590/s1984-29612019057
Aryee EB, Poehlman WFS. (1991). A neural-network-based system to recognize parasites/sealworms on cod fish images. Engineering Applications of Artificial Intelligence 4: 341-350. https://doi.org/10.1016/0952-1976(91)90002-N
Bezerra TN, Decraemer W, Eisendle-Flöckner U, Hodda M, Holovachov O, Leduc D, Miljutin D, Mokievsky V, Peña Santiago R, Sharma J, Smol N, Tchesunov A, Venekey V, Zhao Z, Vanreusel A. (2020). Nemys: World Database of Nematodes. Pseudoterranova decipiens (Krabbe, 1878) Gibson, 1983. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id=123078 on 19 March 2020.
Centers for Disease Control and Prevention [CDC]. (2019) Anasakiasis. Accessed through: DPDx—Laboratory Identification of Parasites of Public Health Concern at https://www.cdc.gov/dpdx/anisakiasis/index.html on 19 March 2020.
Fiorenza EA, Wendt CA, Dobkowski KA, King TL, Pappainou M, Rabinowitz P, Samhouri JF, Wood CL. (2020). It’s a wormy world: Meta-analysis reveals several decades of change in the global abundance of the parasitic nematodes Anisakis spp. and Pseudoterranova spp. in marine fishes and invertebrates. Global Change Biology 26: 2854-2866. https://doi.org/10.1111/gcb.15048
Goater TM, Goater CP, Esch GW. (2014). Parasitism: The diversity and ecology of animal parasites. Cambridge, UK: Cambridge University Press. https://doi.org/10.1017/CBO9781139047876
Hernández-Orts J, Aznar FJ, Blasco-Costa I, García NA, Villora-Montero M, Crespo EA, Raga JA, Montero FE. (2013). Description, microhabitat selection and infection patterns of sealworm larvae (Pseudoterranova decipiens species complex, nematoda: ascaridoidea) in fishes from Patagonia, Argentina. Parasites & Vectors 6: 1-15. https://doi.org/10.1186/1756-3305-6-252
Levsen A, Lunestad BT, Berland B. (2008). Parasites in farmed fish and fishery products. In L. Oyvind, ed. Improving Farmed Fish Quality and Safety, pp. 428-445. Boca Raton, FL: CRC Press. https://doi.org/10.1533/9781845694920.2.428
Ludovisi A, Di Felice G, Carballeda-Sandiao N, Barletta B, Butteroni C, Corinti S, Marucci G, González-Muñoz M, Pozio E, Gómez-Morales MA. (2017). Allergenic activity of Pseudoterranova decipiens (Nematode: Anisakidae) in BALB/c mice. Parasites & Vectors 10: 1-8. https://doi.org/10.1186/s13071-017-2231-4
Malouf AH. (1986). Report of the Royal Commission on Seals and Sealing in Canada. Vol. 3 Pt. 5. Biological Issues. Ottawa, Canada.
Marcogliese DJ. (2001). Review of experimental and natural invertebrate hosts of sealworm (Pseudoterranova decipiens) and its distribution and abundance in macroinvertebrates in eastern Canada. NAMMCO Scientific Publications 3: 27-37. https://doi.org/10.7557/3.2954
McClelland G. (2002). The trouble with sealworms (Pseudoterranova decipiens species complex, Nematoda): a review. Parasitology 124: S183-S203. https://doi.org/10.1017/S0031182002001658
Mercado R, Torres P, Muñoz V, Apt W. (2001). Human infection by Pseudoterranova decipiens (Nematoda, Anisakidae) in Chile: Report of seven cases. Memórias do Instituto Oswaldo Cruz 96: 653-655. https://doi.org/10.1590/S0074-02762001000500010
Murata Y, Ando K, Usui M, Sugiyama H, Hayashi A, Tanemura A, Kato H, Kuriyama N, Kishiwada M, Mizuno S, Saurai H, Isaji S. (2018). A case of hepatic anisakiasis caused by Pseudoterranova decipiens mimicking metastatic liver cancer. BMC Infectious Diseases 18: 1-7. https://doi.org/10.1186/s12879-018-3540-8
Skrzypczak M, Rokicki J, Pawliczka I, Najda K, Dzido J. (2014). Anisakids of seals found on the southern coast of Baltic Sea. Acta Parasitologica 59: 165-172. https://doi.org/10.2478/s11686-014-0226-2
Yu J-R, Seo M, Kim Y-W, Oh M-H, Sohn W-M. (2001). A human case of gastric infection by Pseudoterranova decipiens larva. The Korean Journal of Parasitology 39: 193-196. https://doi.org/10.3347/kjp.2001.39.2.193