The main purpose of this publication is to provide current management practices for chilli thrips in both conventional and organic strawberries. The intended target audience is Extension agents and growers, for whom we hope it will serve as a helpful guide.
Introduction
Strawberry, Fragaria × ananassa Duchesne (Rosales: Rosaceae), production is an important industry in Florida with approximately 11,000 acres under production, generating a value of over $300 million (USDA-NASS 2019). In Florida, the winter strawberry fields are affected by several foliage-, flower-, fruit-, and root-feeding arthropods. Chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), is one such major economically important and relatively new invasive pest of strawberry crops in Florida.
Scirtothrips dorsalis is an invasive and polyphagous pest with a wide host range of 225 plants including vegetable, ornamental, and fruit crops (Kumar et al. 2013). Scirtothrips dorsalis (Figure 1) is native to the Indian subcontinent and was reported to have invaded east Asia in 1981, South Africa in 1986, and Australia in 1998 (Kumar et al. 2013). Since Florida’s sub-tropical climate is conducive for invasive plants and insects, it is not surprising that S. dorsalis could establish and become a pest here (Ferriter et al. 2006; Figure 2). According to USDA-APHIS inspectors, S. dorsalis was intercepted approximately 89 times at several ports between 1984 to 2002, mainly from imported plant materials such as cut flowers, fruits, and vegetables (USDA 2003). In Florida, it was first reported in Okeechobee County in 1991 and in Highlands County in 1994 (Silagyi and Dixon 2006). Since 2004, it has been reported in several counties in Florida and Texas (Kumar et al. 2013).
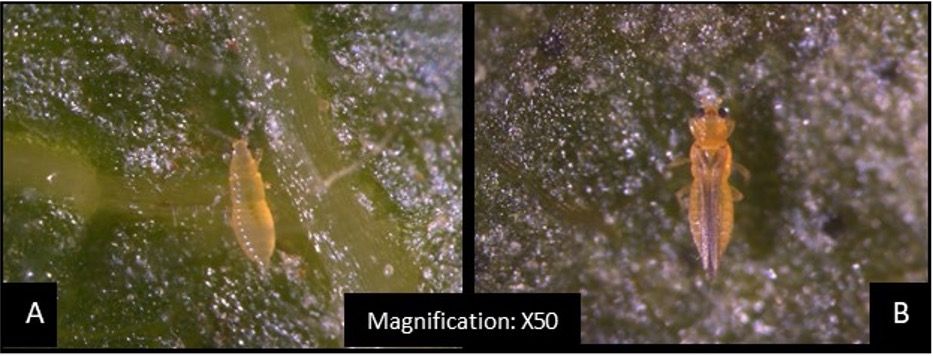
Credit: Gagandeep Kaur
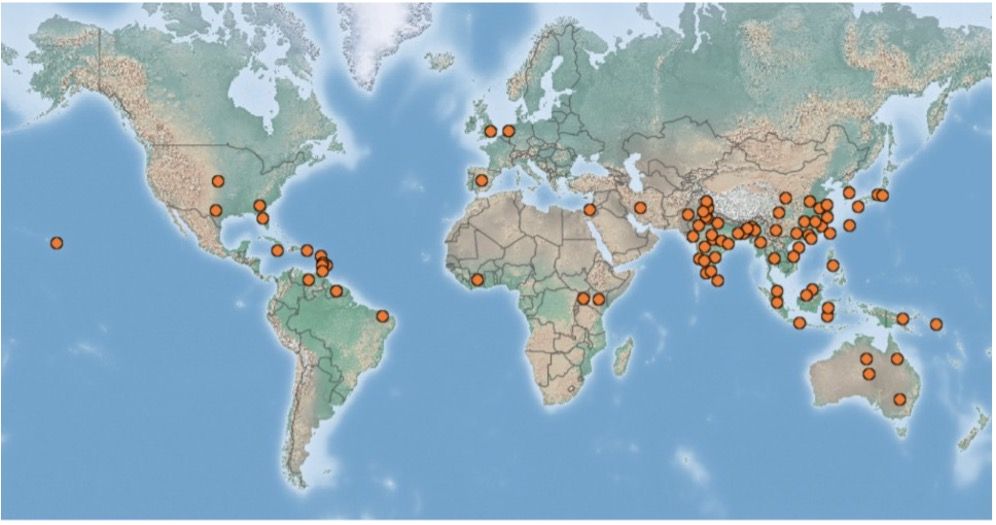
Credit: https://www.cabi.org/isc/datasheet/49065
Morphology and Life Cycle
Scirtothrips dorsalis shows complete metamorphosis, with six life stages: egg, first and second instar larvae, pre-pupa, pupa, and adult stages. Females lay eggs singly by inserting their specially adapted ovipositor into the plant tissue, where the eggs remain protected from predators and insecticide application (Lewis 1973). A single female can lay an average of forty eggs during her lifespan (Tatara 1994). Eggs are very small (0.075 mm), kidney shaped, white, and not visible to the naked eye (Seal et al. 2010). Eggs hatch in 5–8 days depending on temperature and relative humidity. Larvae and adults can typically be found at their feeding site on the mid-vein or borders of the host plant leaves. Larval stages (Figure 3A and 3B) are completed in 8–10 days whereas the pupal stage persists for 2.6–3.3 days (Kumar et al. 2013). The pre-pupa can be identified by its short wing sheaths (Figure 3C and 3D), whereas the pupa has longer wing sheaths that nearly reach the end of the abdomen (Figure 3E and 3F) (Fery et al. Krishna Kumar et al. 1996). Both the pre-pupa and the pupa are sessile, non-feeding stages, and pupae can be found in the leaf litter or in leaf curls of the damaged leaves (Moritz 1997; Kumar et al. 2013, 2014).

Credit: Gagandeep Kaur
Scirtothrips dorsalis adults are very small (<2mm) and yellowish. Scirtothrips dorsalis adults have eight distinctive antennal segments; the head has ocellar setae between posterior ocelli; abdominal tergites have three discal setae in lateral microtrichial fields, and forewings have straight cilia (Figure 4). The wings are dark and fringed, the forewings lighter in color than the hindwings. S. dorsalis males are smaller than females with shorter abdomen (Kumar et al. 2013). Several morphological characteristics that help distinguish S. dorsalis from other thrips (EPPO 2005): pronotum covered with transverse striae, abdominal tergites laterally with many rows of minute microtrichia, sternites and metanotum with setae arising at posterior and anterior margin respectively.
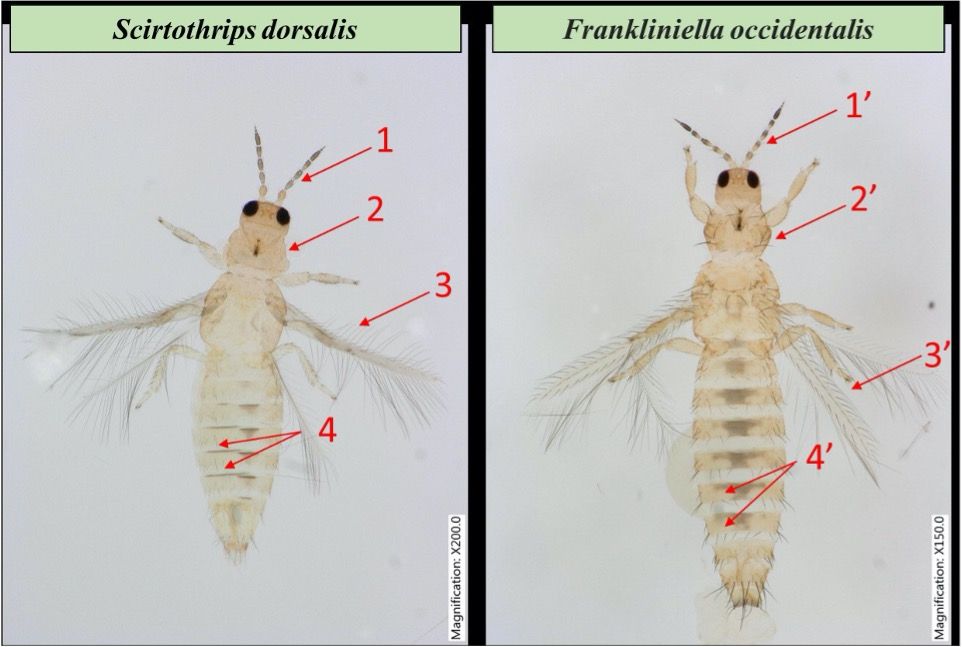
Credit: Gagandeep Kaur
The total life span of S. dorsalis differs depending on host plants, ranging from 11–15.8 days (Seal et al. 2010). Scirtothrips dorsalis is capable of both sexual and asexual reproduction, which contributes to their rapid population growth potential. Asexual reproduction in S. dorsalis occurs when unfertilized eggs develop into males (arrhenotokous parthenogenesis). Eggs fertilized during sexual reproduction develop into both males and females, but males are rare. The typical sex ratio is four females: one male (Dev 1964).
Crop Damage
Scirtothrips dorsalis possess piercing-sucking mouth parts and prefer to feed on younger, nutritious, softer tissues of host plants, such as on meristems or terminals. They do not feed on mature plant parts (Kumar et al. 2013). Scirtothrips dorsalis use their stylet-like mouth parts to penetrate into the young leaves. The wounds they make in the leaves in the process of feeding lead to necrosis and the development of brown to black color (Kumar et al. 2013; Saha et al. 2016). Severe infestation of S. dorsalis can result in complete host plant damage and crop loss.
In strawberries, S. dorsalis starts infesting plants early in the season. Heavy feeding causes reddening and darkening of leaf veins and petioles (Figures 5A, 5B, and 5C). With severe infestations, the entire leaf turns dark, crinkled, and deformed (Figures 5D and 5E). In addition, S. dorsalis feeding on leaves, flowers, and fruits causes leaf distortion, bronzing, and cracking of fruits, which ultimately results in reduced crop yields (Seal et al. 2006; Figures 5F, 5G and 5H).
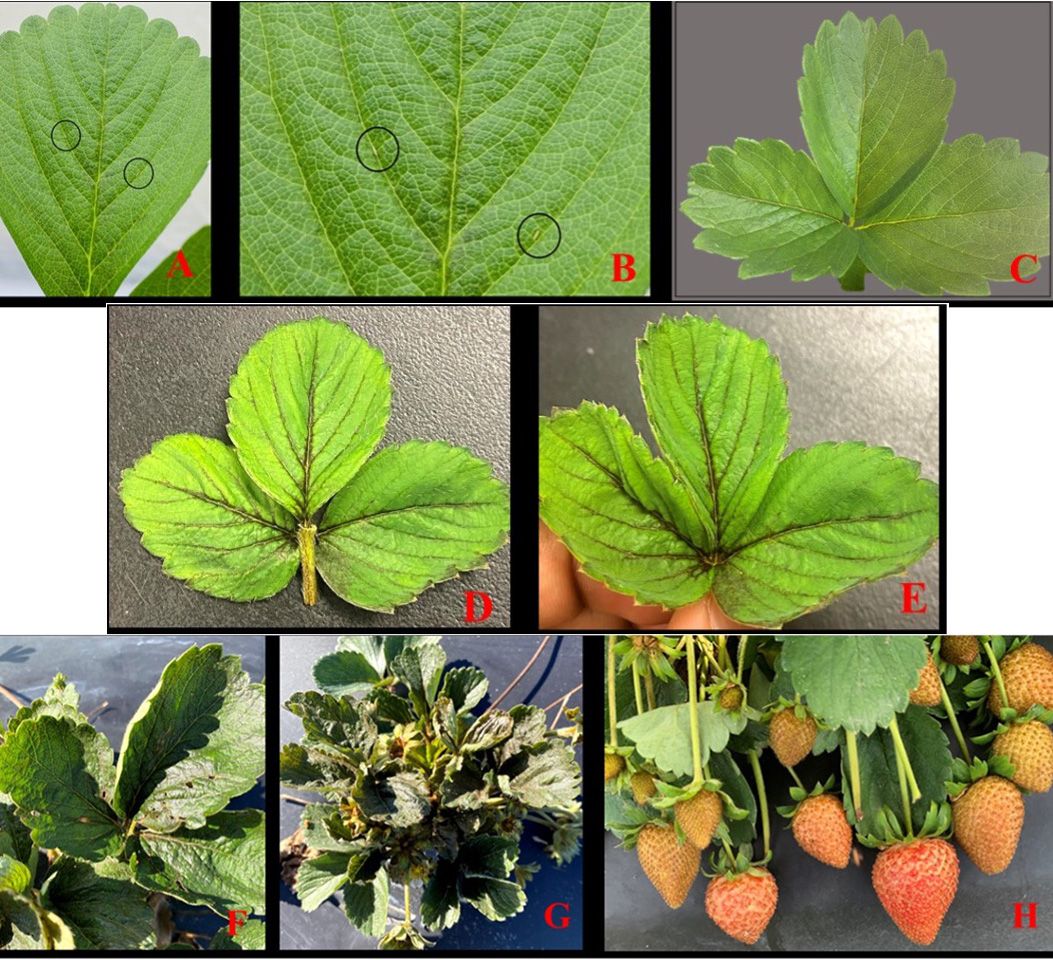
Credit: Gagandeep Kaur
Management
Cultural Control
Field scouting for early detection of S. dorsalis is crucial for management. Scirtothrips dorsalis can be monitored by scouting the fields for injury symptoms. Strawberry leaf trifoliates can also be collected from the fields to count S. dorsalis larvae; however, enough samples should be collected to precisely estimate the S. dorsalis population. Allow up to two weeks to pass after initial detection of S. dorsalis in Florida open-field strawberries before initiating chemical control methods. S. dorsalis tend to aggregate around initial infestation zones within the field (Panthi et al. 2021). Other management practices include removing alternative hosts and weeds (Kumar et al. 2013).
Biological Control
Inoculative augmentation of biological control agents has emerged as a valuable tool in S. dorsalis management, owing to the thrips’ capacity to develop insecticide resistance rapidly. For biological control of S. dorsalis, minute pirate bugs, Orius spp. (Hemiptera: Anthocoridae) and foliar-dwelling entomopathogenic nematodes, Thripinema spp. (Tylenchida: Allantonematidae) have been found effective under controlled conditions and can be integrated into field programs. (Kumar et al. 2013). Minute pirate bugs feed on both larvae and adults whereas entomopathogenic nematodes parasitize thrips females and make them incapable of laying eggs (Funderburk at al. 2007; Seal et al. 2010). Two phytoseiid mites, Neoseiulus cucumeris (Oudemans) and Amblyseius swirskii Athias-Henriot (Arachnida: Phytoseiidae) also provide control of S. dorsalis. Amblyseius swirskii has been found effective for S. dorsalis larval suppression on pepper and strawberry (Arthurs et al. 2009; Lahiri and Yambisa in press). These biological control agents are commercially available for purchase and can be hand-released.
Chemical Control
Currently, the management of S. dorsalis mainly relies on chemical control. Management of S. dorsalis can be challenging for several reasons: They can move to surrounding weed crops to take refuge and lay eggs inside the leaf tissue where the eggs remain protected from pesticide sprays because they are embedded in the leaf. Thrips hide in concealed places such as leaf curls or under fruit calyxes where they can avoid foliar or drench pesticide applications. Several conventional synthetic and OMRI-approved pesticides are labeled for thrips management in Florida strawberry (Lahiri and Panthi 2020; Lahiri and Yambisa in press). Pesticides derived from plant extracts or entomopathogens are most effective if applied 2–3 times within a 5- to 7-day interval.
It is necessary to apply insecticides in rotation to prevent resistance development in S. dorsalis (UF/IFAS Vegetable Production Handbook 2019–2020). Pesticides registered for strawberry crops in Florida should be integrated with other management strategies. Pesticides that can be applied by organic growers are listed in Table 1A; those suitable for conventional growers are listed in Table 1B.
References Cited
Arthurs, S., C. L. McKenzie, J. Chen, M. Dogramaci, M. Brennan, K. Houben, and L. Osborne. 2009. “Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as Biological Control Agents of Chilli Thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on Pepper.” Biological Control. 49:91–96. https://doi.org/10.1016/j.biocontrol.2009.01.002
Dev, H. 1964. “Preliminary Studies on the Biology of the Assam Thrips, Scirtothrips dorsalis Hood on Tea.” Indian Journal of Entomology. 26 (pt. 2).
Ferriter, A., B. Doren, R. Winston, D. Thayer, B. Miller, B. Thomas, M. Barrett, T. Pernas, S. Hardin, and J. Lane. 2006. “The Status of Nonindigenous Species in the South Florida Environment.” South Florida environment report, South Florida Water Management District, Florida Department of Environmental protection. p1–52.
Fery, R. L., and J. M. Schalk. 1991. “Resistance in Pepper (Capsicum annuum L.) to Western Flower Thrips, Frankliniella occidentalis (Pergande).” HortScience. 26:1073–1074. https://doi.org/10.21273/HORTSCI.26.8.1073
Funderburk, Joe, Stan Diffie, Jyotsna Sharma, Amanda Hodges, and Lance Osborne. 2008. “Thrips of Ornamentals in the Southeastern US” ENY-845/IN754. EDIS 2008 (1). https://doi.org/10.32473/edis-in754-2007
Krishna Kumar, K., N. K., M. Aradya, A. A. Deshpande, N. Anand, and P. R. Ramachandar. 1996. “Initial Screening of Chili and Sweet Pepper Germplasm for Resistance to Chili Thrips, Scirtothrips dorsalis Hood.” Euphytica. 89:319–324. https://doi.org/10.1007/BF00022288
Kumar, V., G. Kakkar, C. L. McKenzie, D. R. Seal, and L. S. Osborne. 2013. “An Overview of Chilli Thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) Biology, Distribution and Management.” Weed and Pest Control—Conventional and New Challenges. 53–77. DOI: http://dx.doi.org/10.5772/55045
Jha, Vivek Kumar, Dakshina R. Seal, and Garima Kakkar. 2010. “Chilli Thrips Scirtothrips dorsalis Hood (Insecta: Thysanoptera: Thripidae)”. EDIS 2010 (1). https://doi.org/10.32473/edis-in833-2010
Lahiri, S., and B. Panthi. 2020. “Insecticide Efficacy for Chilli Thrips Management in Strawberry, 2019.” Arthropod Management Tests. 45:tsaa046. https://doi.org/10.1093/amt/tsaa046
Lahiri, S., and A. Yambisa. 2021.“Efficacy of a Biopesticide and Predatory Mite to Manage Chilli Thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in Strawberry.” Florida Entomologist. 104(4):322-324. https://doi.org/10.1653/024.104.0410
Lewis, T. 1973. Thrips, Their Biology, Ecology and Economic Importance. London: Academic. 349 pp.
Mortiz, G. 1997. “Structure, Growth and Development.” Thrips as Crop Pests. 15–63.
Panthi, B. R., J. M. Renkema, S. Lahiri, and O. E. Liburd. 2021. “The Short-Range Movement of Scirtothrips dorsalis (Thysanoptera: Thripidae) and Rate of Spread of Feeding Injury among Strawberry Plants.” Environmental Entomology. 50:12–18. https://doi.org/10.1093/ee/nvaa149
Pugh, A., M. Davis, M. Watson, and T. Withers. 2015. “Exploring Potential Nontarget Impacts of Spinetoram against Beneficial Natural Enemies of Eucalyptus Forests.” New Zealand Plant Protection. 68:438–438. https://doi.org/10.30843/nzpp.2015.68.5839
Saha, D. 2016. “Host Plant-Based Variation in Fitness Traits and Major Detoxifying Enzymes Activity in Scirtothrips dorsalis (Thysanoptera: Thripidae), an Emerging Sucking Pest of Tea.” International Journal of Tropical Insect Science. 36:106–118. https://doi.org/10.1017/S1742758416000102
Seal, D., W. Klassen, and V. Kumar. 2010. “Biological Parameters of Chilli Thrips, Scirtothrips dorsalis Hood, on Selected Hosts.” Environmental Entomology. 39:1389–1398 https://doi.org/10.1603/EN09236
Seal, D., M. Ciomperlik, M. Richards, and W. Klassen. 2006. “Comparative Effectiveness of Chemical Insecticides against the Chilli Thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), on Pepper and Their Compatibility with Natural Enemies.” Crop Protection. 25:949–955. https://doi.org/10.1016/j.cropro.2005.12.008
Silagyi, A., and W. Dixon. 2006. “Assessment of Chili Thrips, Scirtothrips dorsalis Hood. Florida.” Florida Cooperative Agricultural Pest Survey, Program report.
Srivastava, M., L. Bosco, J. Funderburk, and A. Weiss. 2008. “Spinetoram Is Compatible with the Key Natural Enemy of Frankliniella Species Thrips in Pepper.” Plant Health Progress. 9:30. https://doi.org/10.1094/PHP-2008-0118-02-RS
Tatara, A. 1994. “Effect of Temperature and Host Plant on the Development, Fertility and Longevity of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae).” Applied Entomology and Zoology. 29:31–37. https://doi.org/10.1303/aez.29.31
USDA. 2003. “Port Information Network (PIN-309): Quarantine Status Database.” US Department of Agriculture, Animal and Plant Health Inspection Service, Plant Protection and Quarantine, Riverdale, MD, USA.
U.S. Strawberry Industry. 2019. https://www.nass.usda.gov/Publications/Todays_Reports/reports/vegean20.pdf
Whitaker, Vance M., Nathan S. Boyd, Natalia A. Peres, Johan Desaeger, Sriyanka Lahiri, and Peter J. Dittmar. 2020. “2020–2021 Vegetable Production Handbook: Chapter 16. Strawberry Production” HS736/CV134, Rev. 4/2020. EDIS 2020 (VPH). https://doi.org/10.32473/edis-cv134-2020
Table 1A. List of OMRI approved pesticides recommended for S. dorsalis management in strawberry crops in Florida (adopted from UF/IFAS Vegetable Production Handbook of Florida, 2020–2021, Chapter 16 by Whitaker et al. 2020).
Table 1B. List of registered conventional pesticides recommended for S. dorsalis management in strawberry crops in Florida (adopted from UF/IFAS Vegetable Production Handbook of Florida, 2020–2021, Chapter 16 by Whitaker et al. 2020).