Introduction
The purpose of this document is to provide an overview of the current knowledge on the horntail snail (Macrochlamys indica Benson) as well as recommendations to optimize its detection and monitoring in nurseries.
The horntail snail was detected in August 2020 in Miami-Dade County (Talamas 2020). This snail is considered of quarantine importance in the United States because it can become an agricultural pest and potentially host parasitic nematodes of medical significance (Grewal et al. 2003; Cowie et al. 2009; Jayashankar and Murthy 2015). A program to survey, control, and eradicate the horntail snail has been implemented by the Florida Department of Agriculture and Consumer Services, Division of Plant Industry (FDACS-DPI).
Credit: undefined
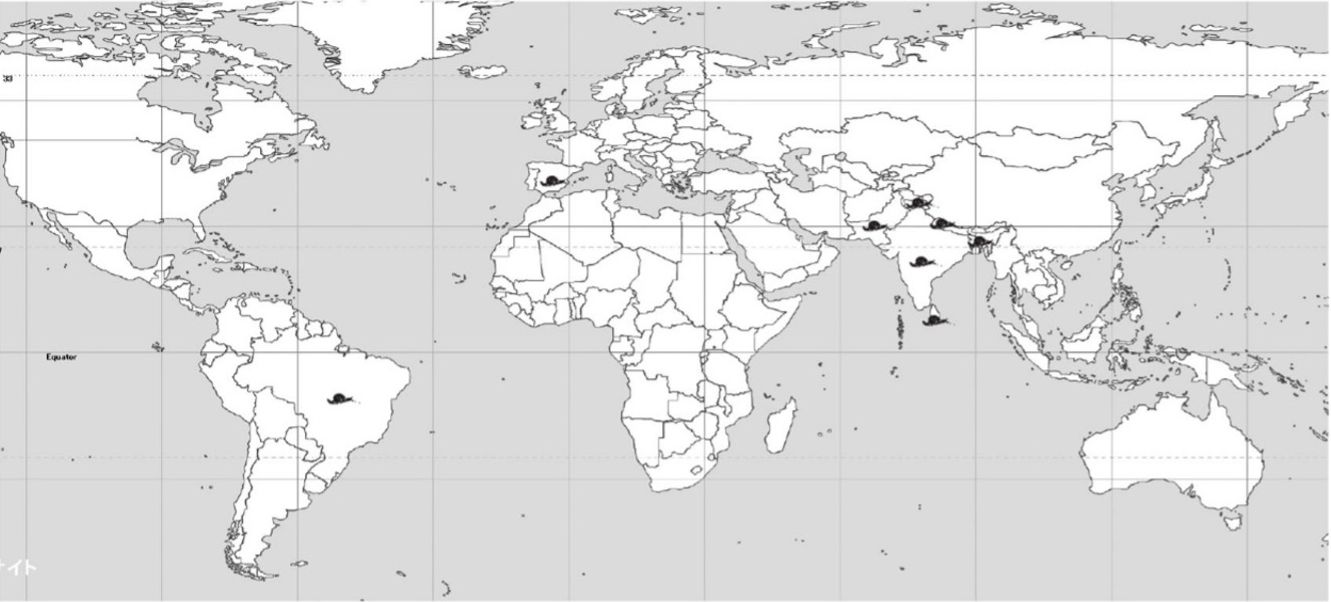
The horntail snail belongs to the genus Macrochlamys (family Ariophantidae), which has over a hundred described species distributed from South to Southeast Asia and southern China (Pholyotha et al. 2018). It occurs in India, Sri Lanka, Bangladesh, Nepal, Pakistan and Brazil (Figure 1) (Raut and Ghose 1984; Biswas et al. 2015; Jayashankar et al. 2015; Agudo-Padron 2018). Jayasshankar et al. (2015) report the occurrence of this snail in Europe; however, the location is not specified, and we were unable to find additional information to substantiate this report. In Bangalore, India, it is known as the common garden snail, and populations are higher in urban settings than in agricultural areas (Jayashankar et al. 2015).
Identification
The horntail snail has a brown body and an amber-colored shell (Figure 2). At the tip of its tail, M. indica has a caudal horn, from which it took its common name (Talamas 2020). The size of adult snails varies with age, and the shell diameter can range from 10 to 21 mm (0.39 – 0.83 in) (Raut and Ghose 1984) (Table 1). A characteristic of this snail species is that it has a flap of flesh that extends backward onto or around the shell (for pictures, see Talamas 2020). In Florida, the jump snail (Ovachlamys fulgens (Gude)) has similar characteristics, such as a caudal horn and similar body and shell coloration. However, this snail has a thinner and more elongated body, and its caudal horn is much larger and thicker (for description and pictures, see Salles et al. 2018).

Credit: FDACS-DPI
Host range and damage
Information is scarce regarding the pest and vector status of the horntail snail in its native range. It feeds mostly on seedlings and fallen, decomposed leaves (Raut and Ghose 1983, 1984; Jayashankar et al. 2015). It is considered a minor pest in some localities in India, where it feeds almost exclusively on moss growing on damp brick walls (Jayashankar et al. 2015). The horntail snail, however, can feed on various plants, including vegetable, fruit, and ornamental crops. In caged studies, preferred vegetables included cucurbits, beans, lettuce, okra, and cabbage, while papaya was the preferred fruit crop. Ornamental plants such as chrysanthemum, china rose hibiscus, marigold, and roses were also considered preferred hosts in caged studies (Raut and Ghose 1983, 1984). In general, younger snails feed on tender plant tissue and avoid the leaf petioles and veins, while adults consume all plant parts (Raut and Ghose 1983).
Biology
The biology of horntail snails was studied under laboratory and field conditions in Bangalore, India. Relative humidity (RH) was found to be the most important factor affecting the population dynamics of this snail species (Raut and Ghose 1979, 1984). When the humidity in the environment is below 46%, the horntail snail enters a dormant state called aestivation. Aestivation varies with geographic latitude and can range from two to eight months. During this period, the snails remain hidden and inactive and lose weight (Raut and Ghose 1979, 1984). In southern India, the horntail snail has an eight-month aestivation period and remains active during the monsoon season. A single individual can live up to four years. Young snails require 130 days to reach sexual maturity. Optimum mating conditions consist of temperatures ranging from 21oC–30oC (69.8oF–86oF) and humidity higher than 86%. Mating usually takes place after rains, and the gestation period ranges from 10–17 days.
Table 1. In India, under field conditions, horntail snails (Macrochlamys indica Benson) of different ages vary in size and oviposition rates (Raut and Ghose 1984). Photo credit: FDACS-DPI.

Credit: undefined

Credit: undefined

Credit: undefined

Credit: undefined
In India, egg laying starts when snails are in their second year (Table 1). Eggs are laid in clutches under loose soil. Adult snails need approximately 10–24 minutes to deposit an egg clutch and, on average, remain in the nest 11 minutes after that (Raut and Ghose 1984). Eggs are round, translucent, and 3.0–3.5 mm (0.12–0.14 in) in size. The number of egg clutches and eggs per clutch varies with the age of the female (see Table 1). The egg clutches are laid at 12 to 49-day intervals, and the egg incubation period ranges from eight to twelve days. After egg hatching, young snails remain immobile for 14–20 hours, and they start feeding on the fourth or fifth day (Raut and Ghose 1984). The snail, however, might not have the same reproductive and feeding habits in new areas of invasion.
Under laboratory conditions with temperature ranging from 21oC to 32oC (69.8oF–89.6oF) and constant 90% RH, the horntail snail's life span ranges from 15 to 19 months, and there is no aestivation. Snails oviposit up to five egg clutches per year with an average of 16 eggs per clutch. Under these conditions, young snails require ten months to reach sexual maturity (Sudhakar 2013).
Dispersal and Environment
Like other land snails, the horntail snail has low natural long-distance dispersal. Human transport of plants and landscape materials provides the major mechanism for introduction of snails into new locations (Bergey et al. 2014). The low long-distance mobility of land snails also offers the opportunity to eradicate local populations (Roda et al. 2016). As in most land snails, active dispersal occurs at night and on cloudy, rainy days (Bailey 1981).
Relative humidity is the most critical factor affecting the biology and behavior of the horntail snail. Temperature ranging from 28oC to 31oC (82.4oF –87.8oF) with a minimum of 19oC (66.2oF) and relative humidity between 85 and 100% are the optimum environmental conditions for the development of this species. Even when the temperature ranges from 9oC to 43oC (48.2oF –109.4oF), the horntail snail can remain active as long as the relative humidity is above 60% (Raut and Ghose 1984).
Association with Human Pathogens
The association between horntail snail and nematodes has not been studied in detail. A single study mentioned an association with non-parasitic Rhabditis sp. nematodes in West Bengal, India (Cowie et al. 2009; Jayashankar and Murthy 2015). However, closely related species, such as Macrochlamys resplendens and the Giant African Snail (Achatina fulica), are known hosts of Angiostrongylus nematodes that can cause eosinophilic meningitis in humans (Grewal et al. 2003). Therefore, the possibility that the horntail snail could host nematodes of medical importance cannot be ruled out.
Recommendations to Optimize Detection and Monitoring of Horntail Snail Populations
Early detection and monitoring of horntail snails in the field are crucial for its successful control/eradication. Recommendations to optimize detection of snail populations include:
- Search for snails at night when they are active, especially after rainfall. Remember that there are also other snails present in our environment. Proper identification is key for a successful management program. FDACS-DPI can assist with snail identification.
- During the day, search for snail trails, shells, and plant damage.
- Search for snails in debris piles, bricks, wall crevices, and near seedlings or succulent plants.
- Search for snails in areas that remain moist and humid, such as near air-conditioning units, pump irrigation systems, and under mulch.
- Train personnel working on ornamental, vegetable, and tropical fruit production on when and how to monitor for snail populations.
In addition, the following actions can be taken to reduce snail populations:
- Improve sanitation and remove hiding places such as boards, stones, debris, weedy areas, leafy branches growing close to the ground, dense ground covers and mulch, and any areas that remain moist.
- If possible, create a less humid environment by adjusting the irrigation frequency and intensity, promptly repairing leaks, and improving drainage.
If you find the horntail snail on your property contact your local county UF/IFAS Extension office (see http://sfyl.ifas.ufl.edu/find-your-local-office/) and the Florida Department of Agriculture and Consumer Services – Division of Plant Industry (FDACS-DPI). For more information from DPI helpline, go to https://www.fdacs.gov/Agriculture-Industry/Pests-and-Diseases/Plant-Pests-and-Diseases/Invasive-Mollusks, call 1-888-397-1517 or email photos to DPIhelpline@FDACS.gov. Properties where the horntail snail has been detected are required to hold orders and quarantine for at least 30 days, sign a compliance agreement, and follow the treatment protocol with metaldehyde (>3.25%) according to FDACS recommendations. For more information, go to the current FDACS Pest Alert document: https://www.fdacs.gov/content/download/93400/file/horntail-snail-pest-alert.pdf. Registered molluscicides for Florida with metaldehyde (>3.25%) for use in nurseries and ornamental crops can be found in Table 2. Note: Do not touch snails with bare hands. Gloves are advised when handling the snail.
Table 2. Molluscicides registered in Florida for use in nurseries and ornamental crops. Products contain more than 3.25% metaldehyde, the minimum percentage recommended by FDACS for eradication of the horntail snail (Macrochlamys indica Benson).
References
Agudo-Padron, A. I. 2018. "Revised And Updated Systematic Inventory of Non-Marine Molluscs Occurring in the State of Santa Catarina/S.C., Central Southern Brazil Region." Advances in Environmental Studies 2:74–81
Bailey, S. E. R. 1981. "Circannual and Circadian Rhythms in the Snail Helix aspersa Müller and the Photoperiodic Control of Annual Activity and Reproduction." Journal of Comparative Physiology 142:89–94. https://doi.org/10.1007/BF00605480
Bergey, E. A., L. L. Figueroa, C. M. Mather, R. J. Martin, E. J. Ray, J. T. Kurien, D. R. Westrop, and P. Suriyawong. 2014. "Trading in Snails: Plant Nurseries as Transport Hubs for Non-Native Species." Biological Invasions 16:1441–1451. https://doi.org/10.1007/s10530-013-0581-1
Biswas, T., B. Tripathy, K. Valarmathi, and S. K. Sajan. 2015. "Taxonomy, Distribution and Conservation of Molluscs in Kangra District of Himachal Pradesh: Three New Records from the State." Ambient Science 2:18–24. https://doi.org/10.21276/ambi.2015.02.2.ra02
Cowie, R. H., R. T. Dillon, D. G. Robinson, and J. W. Smith. 2009. "Alien Non-Marine Snails and Slugs of Priority Quarantine Importance in the United States: A Preliminary Risk Assessment." American Malacological Bulletin 27:113–132. https://doi.org/10.4003/006.027.0210
Grewal, P. S., S. K. Grewal, L. Tan, and B. J. Adams. 2003. "Parasitism of Molluscs by Nematodes: Types of Associations and Evolutionary Trends." Journal of Nematology 35:146–156
Jayashankar, M., and G. S. S. Murthy. 2015. "Record of Gut Associated Nemathelminth in the Giant African Snail Achatina fulica (Bowdich) from Bangalore, India." Journal of Parasitic Diseases 39:144–146. https://doi.org/10.1007/s12639-013-0303-8
Jayashankar, M., M. S. Reddy, and S. Ramakrishna. 2015. "Incidence of the Common Garden Snail, Macrochlamys indica Benson, 1832 (Gastropoda: Ariophantidae) in Bangalore Region." The Bioscan 10:1003–1006
Pholyotha, A., C. Sutcharit, and S. Panha. 2018. "The Land Snail Genus Macrochlamys Gray, 1847 from Thailand, with Descriptions of Five New Species (Pulmonata: Ariophantidae)." Raffles Bulletin of Zoology 7600:763–781
Raut, S. K., K. C. Ghose. 1984. "Pestiferous land snails of India." Zoological Survey of India 1–151
Raut, S. K., and K. C. Ghose. 1983. "Food Preference and Feeding Behaviour of Two Pestiferous Snails, Achatina fulica Bowdich and Macrochlamys indica Godwin-Austen." Records of the Zoological Survey of India 80:421–440
Raut, S. K., and K. C. Ghose. 1979. "Factors Influencing Mortality in Land Snails, Achatina fulica Bowdich and Macrochlamys indica Godwin-Austen during Aestivation." Proceedings of the Zoological Society of London 32:107–120
Roda A, G. Nachman, S. Weihman, M. Y. Cong, and F. Zimmerman. 2016. "Reproductive Ecology of the Giant African Snail in South Florida: Implications for Eradication Programs." PLoS One 11:1–18. https://doi.org/10.1371/journal.pone.0165408
Salles, A. C. A., C. D. C. Oliveira, and R. S. Absalão. 2018. "Redescription of the Jumping Snail Ovachlamys fulgens (Glide, 1900) (Gastropoda: Helicarionoidea: Helicarionidae): An Anatomical and Conchological Approach." Nautilus (Philadelphia) 132:19–29
Sudhakar, B. S. 2013. "Chapter 3, Life Cycle of Macrochlamys indica." Effect of thiamethoxam and diafenthiuron on land snail Macrochlamys indica (Bensonpulmonata: Ariophantidae). Ph.D. Dissertation, North Maharashtra University: 42–75
Talamas, E. J. 2020. Pest Alert: Horntail Snail, Macrochlamys indica Benson.