Introduction
West Nile virus (WNV) is an arbovirus (arthropod-borne virus) transmitted to humans and equines via mosquito bites and a substantial concern to public and veterinary health. West Nile virus was introduced to the United States in 1999, and with over 52,000 reported cases of human infections, it is the most frequent cause of mosquito-borne disease in the continental United States (CDC 2022). Many mosquito species can transmit WNV in the United States, and many wildlife host species can become infected with the virus and transmit it to biting mosquitoes. The many potential hosts and vectors make identifying high risk areas or time periods a complex task.
West Nile virus harms the equine industry, domestic pets, and livestock in the United States. (Bosco-Lauth and Bowen 2019). Multiple equine WNV epizootics (animal outbreaks) have occurred since 1999, with more than 30,000 equine cases reported in the United States (Bosco-Lauth and Bowen 2019). In Florida, a total of 460 WNV infections in people and 757 WNV infections in horses and other equines have been reported between 2001 and 2021 (CDC 2022; FDOH 2022). This publication is intended to provide information about WNV to researchers and stakeholders in mosquito control and public health professions, as well as to the general public.
What is West Nile virus?
West Nile virus naturally circulates in the environment between mosquito vectors and birds, occasionally spilling over (transferring) to humans and equines through the bite of an infected mosquito. The virus is a member of the genus Flavivirus, which also includes other arboviruses that are important to human health, such as dengue virus (DENV), yellow-fever virus (YFV), Zika virus (ZIKV), and Saint Louis encephalitis virus (SLEV). The virus is considered endemic, or reported regularly, throughout the United States with locally acquired human infections reported consistently each year (Figure 1) (Lanciotti et al. 1999; Reisen 2013).
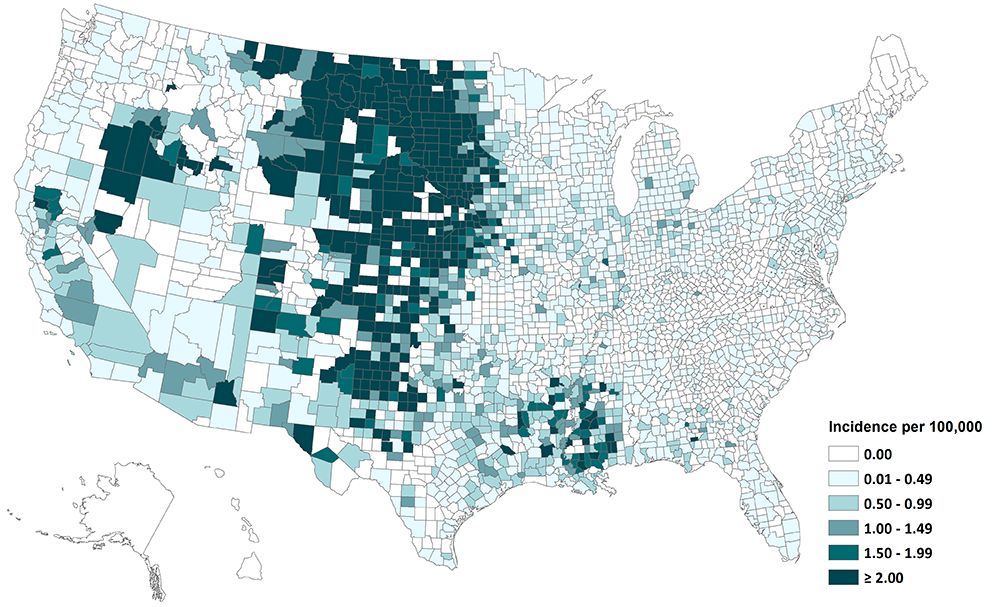
Credit: CDC 2022
Signs and Symptoms
Although most people infected with WNV are asymptomatic and do not develop noticeable symptoms, 1 out of 5 infected individuals develop flu-like symptoms and can take weeks to recover (CDC 2022). The most common signs and symptoms include fever, swollen lymph glands, nausea, vomiting, muscle aches, joint pain, and rashes (Zou et al. 2010). A smaller percentage of the population infected with WNV (1 out of 150) may develop neuroinvasive disease that leads to encephalitis (inflammation of the brain) or meningitis (inflammation of the membranes covering the brain and spinal cord). Approximately 10% of these neuroinvasive disease cases result in death (Petersen et al. 2013). Although this severe form of the disease can occur across all ages, it is more likely to develop in people over 60 years of age, among which the risk may approach 1 out of 50 infected individuals (Carson et al. 2012).
Diagnosis
Individuals who develop symptoms may seek help from a healthcare provider who can order tests to diagnose WNV infection (CDC 2022). Serological or other molecular tests such as viral isolation and reverse transcriptase PCR are often used to determine whether or not an individual has antibodies to the virus (indicating a past WNV infection) or the actual viral particles circulating in the system (Zou et al. 2010). WNV is a nationally notifiable disease, and all positive infections are reported to the US Centers for Disease Control and Prevention (CDC). Because WNV can be transmitted through blood transfusions, all blood donations are tested for the virus, and positive infections are reported to CDC.
Treatment
There are currently no medications available that are specific for treating a WNV infection. Over-the-counter pain relievers may be used to reduce fever and relieve some of the symptoms associated with WNV infection. Patients who develop neuroinvasive disease require hospitalization and supportive treatment (CDC 2022; Petersen et al. 2013).
Challenges
Most WNV infections are not reported because approximately only 20% of infected people will develop symptoms, and symptoms of WNV disease are often confused with more common viruses or are similar to other medically important arboviruses (CDC 2022). Serological tests can be misleading at times when WNV and other flaviviruses such as DENV and SLEV can also be present (Petersen et al. 2013). Collectively, these factors lead to underreporting and underestimation of WNV prevalence.
Prevention
Although four effective WNV equine vaccines are available, a need continues for a human-safe vaccine (Kaiser and Barrett 2019). The most effective way to prevent WNV infection currently is to avoid mosquito bites. Wearing clothes that cover exposed skin—for example, long pants and long-sleeved shirts, applying mosquito repellent when outdoors, limiting outdoor activities to daylight hours or away from peak mosquito biting times, and installing screens in windows are some of the most commonly recommended practices to prevent arboviral infections like WNV.
Veterinary Health
Companion animals, livestock, and, most importantly, equines, are also at risk of WNV infections in the United States. (Bosco-Lauth and Bowen 2019). Similarly to human WNV infections, 10% of infected horses will develop neuroinvasive symptoms, which can include high fever, impaired coordination, paresis (partial paralysis), and tremors (Ostlund et al. 2001). Because WNV is endemic to Florida, equine vaccinations against WNV are necessary, and owners are encouraged to administer the vaccine in a 2-dose initial treatment followed by an annual booster (AAEP 2022, Bosco-Lauth and Bowen 2019). Currently, four effective WNV equine vaccines are available(Kaiser and Barrett 2019).
Transmission Cycle
The transmission cycle of WNV is very complex and involves multiple components, including mosquito vectors, vertebrate hosts, and the environment. Multiple modes of transmission exist for WNV, including blood transfusions and organ transplantation between humans, and vertical transmission from female mosquitoes to their eggs. However, the primary route of infection occurs when infected female mosquitoes take a blood meal from vertebrate hosts. Although WNV has been found within multiple mosquito species, the transmission cycle involves relatively few species serving as primary vectors (vectors that efficiently spread WNV among bird populations) or bridge vectors (vectors that transfer WNV from circulation among birds to mammals or other vertebrates) (Figure 2) (Turell et al. 2005; Rochlin et al. 2019). Competent mosquito vectors, often in the genus Culex, that primarily feed on avian hosts, acquire WNV during blood feeding from infected birds, which often exhibit high levels of virus circulating in their bloodstream (viremia) (Colpitts et al. 2012; Reisen 2013). After the female ingests WNV during blood feeding, it takes approximately 2–14 days before that mosquito can transmit WNV to a new host. The period varies by mosquito species and is influenced by temperature (Sardelis et al. 2001; Turell et al. 2002; Kilpatrick et al. 2007). When infected WNV mosquito vectors take blood meals from humans, horses, and other mammals, transmission can occur. This is referred to as a spillover event to a “dead end” host because these mammals do not develop high levels of viremia, making it unlikely that mosquitoes feeding on those infected animals will become infected with WNV (Figure 2) (Godsey et al. 2005b; Ronca et al. 2021).
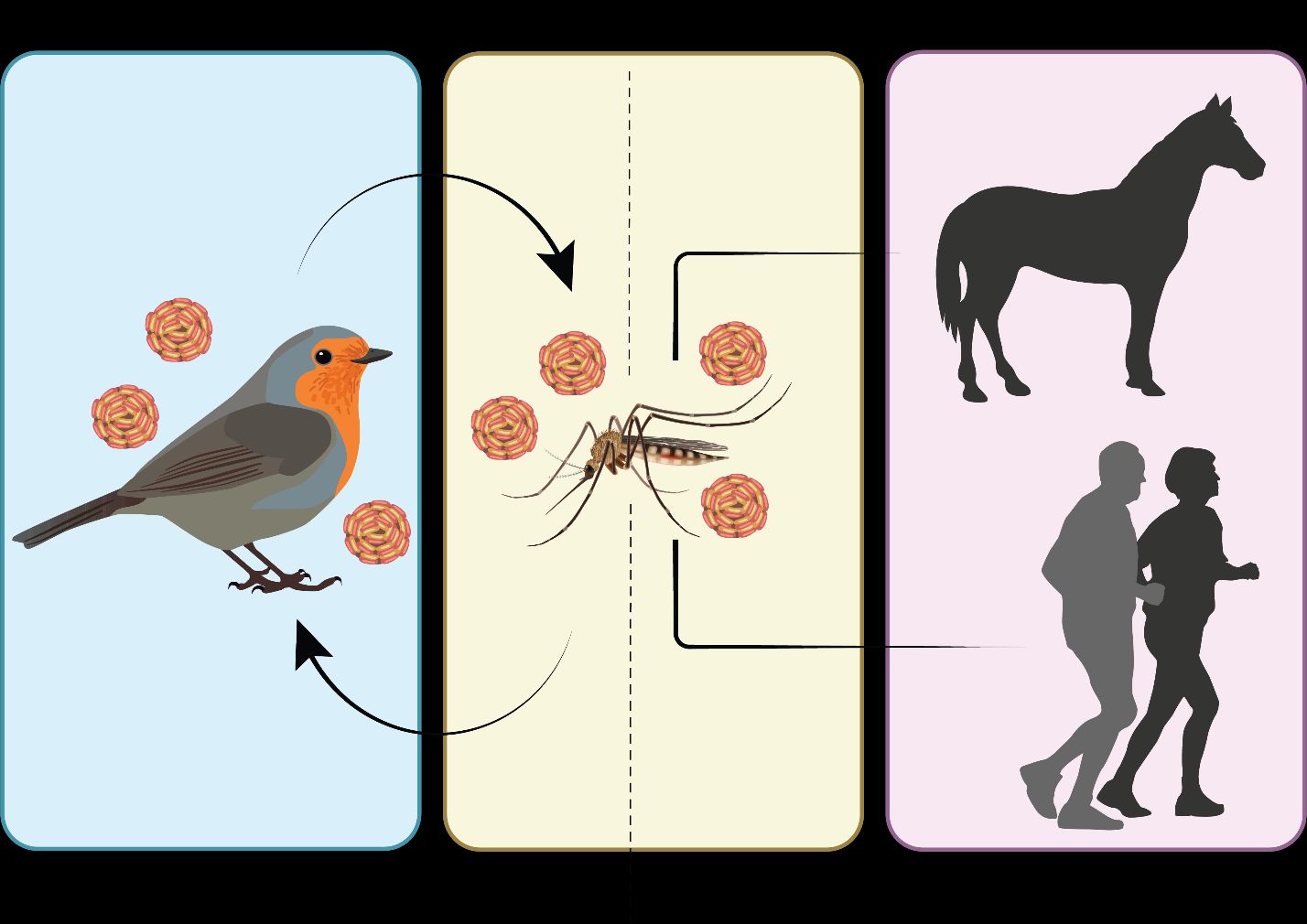
Credit: undefined
Different mosquito species can be responsible for WNV transmission depending on the season or the location (Reisen 2013; Rochlin et al. 2019). Mosquitoes from the Culex subgenus Culex are the most important vectors for WNV. In Florida, the mosquito species Culex quinquefasciatus Say (Figure 3) and Culex nigripalpus (Figure 4) Theobald are considered the most important mosquito species for WNV transmission (Godsey et al. 2005a; Kilpatrick et al. 2006).

Credit: Lawrence Reeves, UF/IFAS

Credit: Lawrence Reeves, UF/IFAS
Culex quinquefasciatus are found more commonly in urban areas. The females of this species prefer semi-permanent freshwater habitats to lay their eggs. In urban and agricultural settings, such habitats include unmaintained swimming pools and a variety of catch basins, retention ponds, or artificial containers that are rich in organic material (Day et al. 2015; Hill and Connelly 2019; Rochlin et al. 2019). Culex nigripalpus tends to be found in mixed vegetative semi-urban and rural habitats since the females prefer to lay eggs in freshly flooded roadside ditches or agricultural furrows. They are also known to oviposit in a variety of aquatic habitats, including artificial containers (Day 2004; Rey et al. 2006). Culex quinquefasciatus plays an important role in maintaining the virus within bird populations, referred to as the enzootic cycle, and is capable of serving as a bridge vector, transmitting the virus to humans or other dead-end hosts (Saleem and Lobanova 2020). This mosquito species is considered the primary vector of WNV in the southeast region of the United States. In Florida, however, it is unclear whether this species plays a more important role than Culex nigripalpus in WNV transmission (Sardelis et al. 2001; Godsey et al. 2005b; Vitek et al. 2008; Day et al. 2015; Rochlin et al. 2019).
In Florida, Culex nigripalpus undergoes a seasonal change in its host use. Culex nigripalpus feeds primarily from birds in the winter and spring but shifts to feeding primarily from mammals in the summer and fall (Edman and Taylor 1968). This seasonal change is thought to drive the timing of amplification (increase in transmission) of WNV among populations of wild birds and spillover to humans and other dead-end hosts.
In the western United States, Culex tarsalis (Figure 5) is also an important WNV vector in addition to Culex quinquefasciatus, and Cx. tarsalis is also found in Florida. Like Culex nigripalpus, Culex tarsalis undergoes a seasonal shift in its host use, feeding primarily from birds early in the year and shifting toward mammals later in the year (Tempelis et al. 1965).

Credit: Lawrence Reeves, UF/IFAS
West Nile virus has been detected in multiple vertebrate animals ranging from reptiles to mammals, but avian species are considered to be the main hosts because of their ability to become infected with and amplify the virus for subsequent transmission (Komar 2000; Klenk and Komar 2003; Colpitts et al. 2012; Reisen 2013; Taieb et al. 2020). In Florida, several songbirds that are known to be competent WNV amplifying hosts are distributed throughout all or parts of the state according to their residential distributions or overwintering routes (Foppa et al. 2011; eBird 2021). These include mourning doves (Zenaida macroura), common grackles (Quiscalus quiscula), northern cardinals (Cardinalis cardinalis), and the endangered Florida scrub jays (Aphelocoma coerulescens) (Kilpatrick et al. 2007; Kain and Bolker 2019). Migratory birds may play an important role in WNV transmission in Florida, as they often congregate in great numbers and occupy the same area for a period of time in the winter and early spring months (Shaman et al. 2005; Day et al. 2015; eBird 2021).
What are the chances of becoming infected with WNV?
While WNV peak infection rate in birds is in late spring and early summer, human outbreaks usually happen in late summer and early fall. This time lag in the observed high proportions of infected avian amplifying hosts and mammalian dead-end hosts is assumed to be connected by the vertebrate immunization time (the time it takes for a large proportion of wild vertebrate hosts to become immunized to WNV via previous infection), as well as the mosquito abundance and host use (Edman and Taylor 1968; DeGroote et al. 2012). The risk of WNV transmission to humans generally is greatest from mid to late summer in Florida and much of the conterminous United States (Figure 6) (Petersen et al. 2013).
Overall, the number of human WNV cases in Florida is low; however, periodic outbreaks occurring in 2003, 2012, 2018, and 2020 highlight the need for continued vigilance to protect against mosquito bites that may result in transmission (Table 1).
Table 1. Human cases of WNV and neuroinvasive disease during outbreak years in Florida.
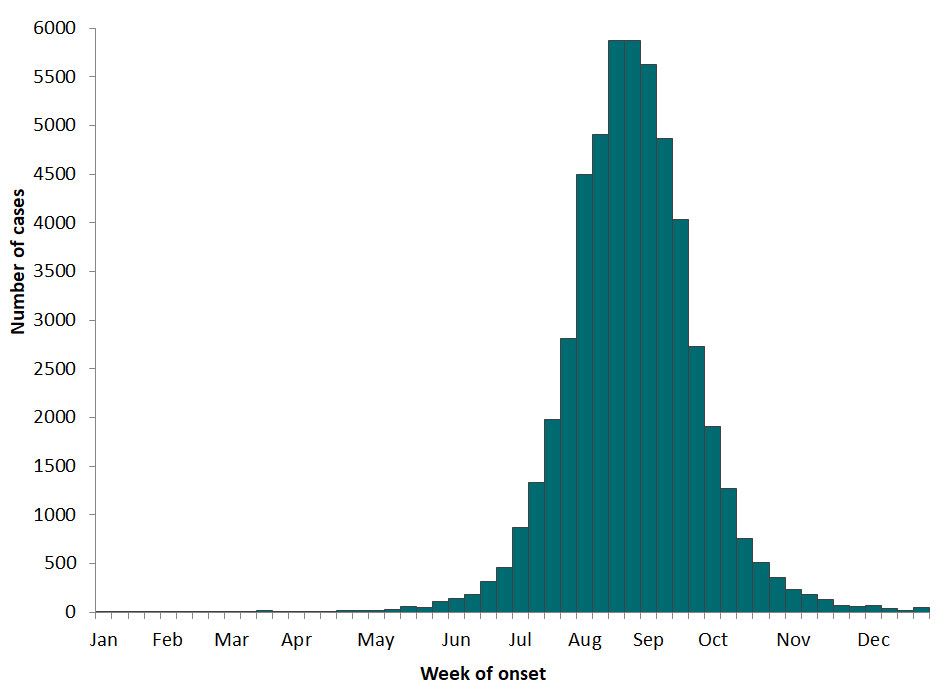
Credit: CDC 2022
Additional Resources
The following State of Florida resources provide additional information about WNV human and equine health:
- Florida Department of Health - https://www.floridahealth.gov/%5C/diseases-and-conditions/west-nile-virus/index.html
- Florida Department of Agriculture and Consumer Services -https://www.fdacs.gov/Agriculture-Industry/Pests-and-Diseases/Animal-Pests-and-Diseases/West-Nile-Virus
References
American Association of Equine Practitioners (AAEP). 2022. West Nile Virus. Vaccines. https://aaep.org/guidelines/vaccination-guidelines/core-vaccination-guidelines/west-nile-virus#:~:text=The%20vaccine%20contains%20an%20adjuvant,disease%2C%20viremia%2C%20and%20encephalitis. Accessed on 19 Jul 2022.
Bosco-Lauth, A. M., and R. A. Bowen. 2019. West Nile Virus: Veterinary Health And Vaccine Development. Journal of Medical Entomology 56:1463–1466. https://doi.org/10.1093/jme/tjz125
Carson, P. J., S. M. Borchardt, B. Custer, H. E. Prince, J. Dunn-Williams, V. Winkelman, L. Tobler, B. J. Biggerstaff, R. Lanciotti, L. R. Petersen, and M. P. Busch. 2012. Neuroinvasive Disease and West Nile Virus Infection, North Dakota, USA, 1999–2008.” Emerging Infectious Diseases 18 (4): 684–686. https://doi.org/10.3201/eid1804.111313
Centers for Disease Control and Prevention (CDC). 2022. West Nile virus disease. https://www.cdc.gov/westnile/index.html. Accessed on 09 May 2022.
Colpitts, T. M., M. J. Conway, R. R. Montgomery, and E. Fikrig. 2012. “West Nile Virus: Biology, Transmission, and Human Infection.” Clinical Microbiology Reviews 25:635–648. https://doi.org/10.1128/CMR.00045-12
Day, J. F. 2004. “The Florida SLE mosquito, Culex (Culex) nigripalpus Theobald (Insecta: Diptera: Culicidae).” EDIS 2004. https://edis.ifas.ufl.edu/publication/IN136
Day, J. F., W. J. Tabachnick, and C. T. Smartt. 2015. “Factors That Influence the Transmission of West Nile Virus in Florida.” Journal of Medical Entomology. 52:743–754. https://doi.org/10.1093/jme/tjv076
DeGroote, J. P., and R. Sugumaran. 2012. “National and Regional Associations between Human West Nile Virus Incidence and Demographic, Landscape, and Land Use Conditions in the Coterminous United States.” Vector-Borne Zoonotic Diseases. 12 (8): 657–65. https://doi.org/10.1089/vbz.2011.0786
eBird. 2021. The Cornell Lab of Ornithology. https://ebird.org/home. Accessed on 09 May 2022.
Edman, J. D., and D. J. Taylor. 1968. “Culex nigripalpus: Seasonal Shift in the Bird-Mammal Feeding Ratio in a Mosquito Vector of Human Encephalitis.” Science 161: 67–68. https://doi.org/10.1126/science.161.3836.67
Florida Department of Health (FDOH). 2022. West Nile Virus (WNV). https://www.floridahealth.gov/%5C/diseases-and-conditions/west-nile-virus/index.html. Accessed on 19 Jul 2022.
Foppa, I. M., R. H. Beard, and I. H. Mendenhall. 2011. The Impact of West Nile Virus on the Abundance of Selected North American Birds.” BMC Veterinary Research 7:43. https://doi.org/10.1186/1746-6148-7-43
Godsey, M. S., R. Nasci, H. M. Savage, S. Aspen, R. King, A. M. Powers, et al. 2005a. “West Nile Virus-Infected Mosquitoes, Louisiana, 2002.” Emerging Infectious Diseases Journal 11:1399–1404. https://doi.org/10.3201/eid1109.040443
Godsey, M. S. Jr, R. Nasci, H. M. Savage, et al. 2005. “West Nile Virus-Infected Mosquitoes, Louisiana, 2002.” Emerging Infectious Diseases Journal11 (9): 1399–1404. https://doi.org/10.3201/eid1109.040443
Godsey, M. S., M. S. Blackmore, N. A. Panella, K. Burkhalter, K. Gottfried, L. A. Halsey, R. Rutledge, et al. 2005b. “West Nile Virus Epizootiology in the Southeastern United States, 2001.” Vector-Borne Zoonotic Diseases 5:82–89. https://doi.org/10.1089/vbz.2005.5.82
Hill, S., and R. Connelly. 2019. “Southern House Mosquito, Culex (Culex) quinquefasciatus Say (Insecta: Diptera: Culicidae).” EDIS 2019. https://edis.ifas.ufl.edu/publication/IN837
Kain, M. P., and B. M. Bolker. 2019. “Predicting West Nile Virus Transmission in North American Bird Communities Using Phylogenetic Mixed Effects Models and eBird Citizen Science Data.” Parasites & vectors 12 (1): 395. https://doi.org/10.1186/s13071-019-3656-8
Kaiser, J. A., and A. D. T. Barrett. 2019. “Twenty Years of Progress toward West Nile Virus Vaccine Development.” Viruses 11 (9): 823. https://doi.org/10.3390/v11090823
Kilpatrick, A. M., L. D. Kramer, M. J. Jones, P. P. Marra, and P. Daszak. 2006. “West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding Behavior.” PLOS Biology 4:e82. https://doi.org/10.1371/journal.pbio.0040082
Kilpatrick, A. M., S. L. LaDeau, and P. P. Marra. 2007. “Ecology of West Nile Virus Transmission and Its Impact on Birds in the Western Hemisphere” The Auk 124 (4): 1121–1136. https://doi.org/10.1093/auk/124.4.1121
Klenk, K. and N. Komar. 2003. “Poor Replication of West Nile Virus (New York 1999 Strain) in Three Reptilian and One Amphibian Species.” The American Journal of Tropical Medicine and Hygiene 69 (3): 260–2. https://doi.org/10.4269/ajtmh.2003.69.260
Komar, N. 2000. “West Nile Viral Encephalitis.” Revue Scientifique et Technique 19 (1): 166–76. https://doi.org/10.20506/rst.19.1.1201
Lanciotti, R., J. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K. Volpe, M. Crabtree, and J. Scherret. 1999. “Origin of the West Nile Virus Responsible for an Outbreak of Encephalitis in the Northeastern United States.” Science 286:2333–2337. https://doi.org/10.1126/science.286.5448.2333
Ostlund, E. N., R. L. Crom, D. D. Pedersen, D. J. Johnson, W. O. Williams, and B. J. Schmitt. 2001. “Equine West Nile Encephalitis, United States.” Emerging Infectious Diseases Journal (4): 665–9. https://doi.org/10.3201/eid0704.017412
Petersen, L. R., A. C. Brault, and R. S. Nasci. 2013. “West Nile Virus: Review of the Literature.” JAMA 310 (3): 308–315. https://doi.org/10.1001/jama.2013.8042
Reisen, W. K. 2013. “Ecology of West Nile Virus in North America.” Viruses 5 (9): 2079–2105. https://doi.org/10.3390/v5092079
Rey, J. R., N. Nishimura, B. Wagner, M. A. Braks, S. M. O'Connell, and L. P. Lounibos. 2006. “Habitat Segregation of Mosquito Arbovirus Vectors in South Florida.” Journal of Medical Entomology 43 (6): 1134–41. https://doi.org/10.1093/jmedent/43.6.1134
Ronca, S. E., J. C. Ruff, and K. O. Murray. 2021. “A 20-year historical review of West Nile virus since its initial emergence in North America: Has West Nile virus become a neglected tropical disease?” PLoS Neglected Tropical Diseases 15:e0009190. https://doi.org/10.1371/journal.pntd.0009190
Rochlin, I., A. Faraji, K. Healy, and T. G. Andreadis. 2019. “West Nile Virus Mosquito Vectors in North America.” Journal of Medical Entomology 56:1475–1490. https://doi.org/10.1093/jme/tjz146
Saleem, M. A., and I. Lobanova. 2020. “Mosquito-Borne Diseases.” Dengue Virus Disease. From Origin to Outbreak. Pages 57–83. https://doi.org/10.1016/B978-0-12-818270-3.00005-9
Sardelis, M. R., M. J. Turell, D. J. Dohm, and M. L. O'Guinn. 2001. “Vector Competence of Selected North American Culex and Coquillettidia Mosquitoes for West Nile Virus.” Emerging Infectious Disease Journal 7:1018. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2631924/
Shaman, J., J. F. Day, and M. Stieglitz. 2005. “Drought-Induced Amplification and Epidemic Transmission of West Nile Virus in Southern Florida.” Journal of Medical Entomology 42:134–141. https://doi.org/10.1093/jmedent/42.2.134
Taieb, L., A. Ludwig, N. H. Ogden, R. L. Lindsay, M. Iranpour, C. A. Gagnon, and D. J. Bicout. 2020. “Bird Species Involved in West Nile Virus Epidemiological Cycle in Southern Québec.” International Journal of Environmental Research and Public Health 17:4517. https://doi.org/10.3390/ijerph17124517
Tempelis, C. H., W. C. Reeves, R. E. Bellamy, and M. F. Lofy. 1965. “A Three-Year Study of the Feeding Habits of Culex tarsalis in Kern County, California.” The American Journal of Tropical Medicine and Hygiene 14:170–177. https://doi.org/10.4269/ajtmh.1965.14.170
Turell, M. J., D. J. Dohm, M. R. Sardelis, M. L. O’guinn, T. G. Andreadis, and J. A. Blow. 2005. “An Update on the Potential of North American Mosquitoes (Diptera: Culicidae) to Transmit West Nile Virus.” Journal of Medical Entomology 42:57–62. https://doi.org/10.1093/jmedent/42.1.57
Turell, M. J., M. R. Sardelis, M. L. O’Guinn, and D. J. Dohm. 2002. “Potential Vectors of West Nile Virus in North America.” In Japanese Encephalitis and West Nile Viruses edited by J. Mackenzie, A. Barrett, and V. Deubel. Springer-Verlag, Berlin 241–252. https://doi.org/10.1007/978-3-642-59403-8_12
Vitek, C. J., S. L. Richards, C. N. Mores, J. F. Day, and C. C. Lord. 2008. “Arbovirus Transmission by Culex nigripalpus in Florida, 2005.” Journal of Medical Entomology 45:483–493. https://doi.org/10.1093/jmedent/45.3.483
Zou, S., G. A. Foster, R. Y. Dodd, L. R. Petersen, and S. L. Stramer. 2010. “West Nile Fever Characteristics among Viremic Persons Identified through Blood Donor Screening.” The Journal of Infectious Diseases 202 (9): 1354–1361. https://doi.org/10.1086/656602