Introduction
This publication summarizes available information about the Keystone virus (KEYV), a neglected mosquito-borne virus of the southeastern United States. Keystone virus is a negative-sense single-stranded RNA arthropod-borne virus (arbovirus) belonging to the genus Orthobunyavirus in the family Peribunyaviridae. It is the only member of the species Keystone orthobunyavirus and was previously classified as a member of a group of viruses traditionally known as the California serogroup arboviruses (Henry et al. 2022) that included La Crosse virus, Jamestown Canyon virus, Snowshoe hare virus, California encephalitis virus, and Trivittatus virus. All of the aforementioned viruses can be transmitted to humans by the bite of an infected mosquito vector. Keystone virus was first described after it had been isolated from field-collected adult mosquitoes of the species Aedes atlanticus in the Tampa Bay area of Florida during a surveillance program for encephalitis-causing arboviruses in 1964 (Bond et al. 1966). Although KEYV was isolated from several other mosquito species, including Ae. infirmatus, Ae. taeniorhynchus, and Culex (Melanoconion) species, Ae. atlanticus is considered to be the primary vector (Sudia et al. 1971; Evans and Peterson 2019). Earlier ecological studies conducted during the 1960s and 1970s indicated that the geographic distribution of KEYV is widespread in the southeastern United States, occurring in a sylvan cycle (circulating in natural ecosystems), which happens between woodland mosquitoes and vertebrate wildlife hosts (Fine and LeDuc 1978). Occasionally, arboviruses like KEYV that circulate in sylvan transmission cycles can “spill over” to infect humans. Zoonotic spillover occurs when an animal pathogen establishes an infection in a human (Plowright et al. 2017). For mosquito-borne arboviruses, the bite from an infected mosquito is the main route by which zoonotic spillover occurs. Few data are available on the frequency of zoonotic spillover events with KEYV, but serological surveys (tests to detect the presence of KEYV antibodies in blood samples, indicating previous infection with KEYV) of the Tampa Bay Area, Florida, found that about 20% of people living in that region had been previously infected with KEYV (Parkin et al. 1972). Keystone virus was recently isolated from a human for the first time, from a teenage male patient in north-central Florida in 2018 who exhibited symptoms that included a rash and fever (Lednicky et al. 2018). It is not yet known whether KEYV can be spread by means of transmission other than the bite of an infected mosquito (e.g., perinatal and/or sexual transmission), as has been observed with other arboviruses (e.g., Zika virus). If transmission by other means does occur, it may increase the public health threat of KEYV in the southeastern United States.
Distribution
Keystone virus has wide distribution in the southeastern United States (Bond et al. 1966; Sudia et al. 1971; Wills et al. 1974; LeDuc et al. 1975a; Watts et al. 1988; Lednicky et al. 2018). It has been detected in mosquitoes or vertebrate wildlife hosts as far west as Texas and as far north as Pennsylvania (Figure 1).
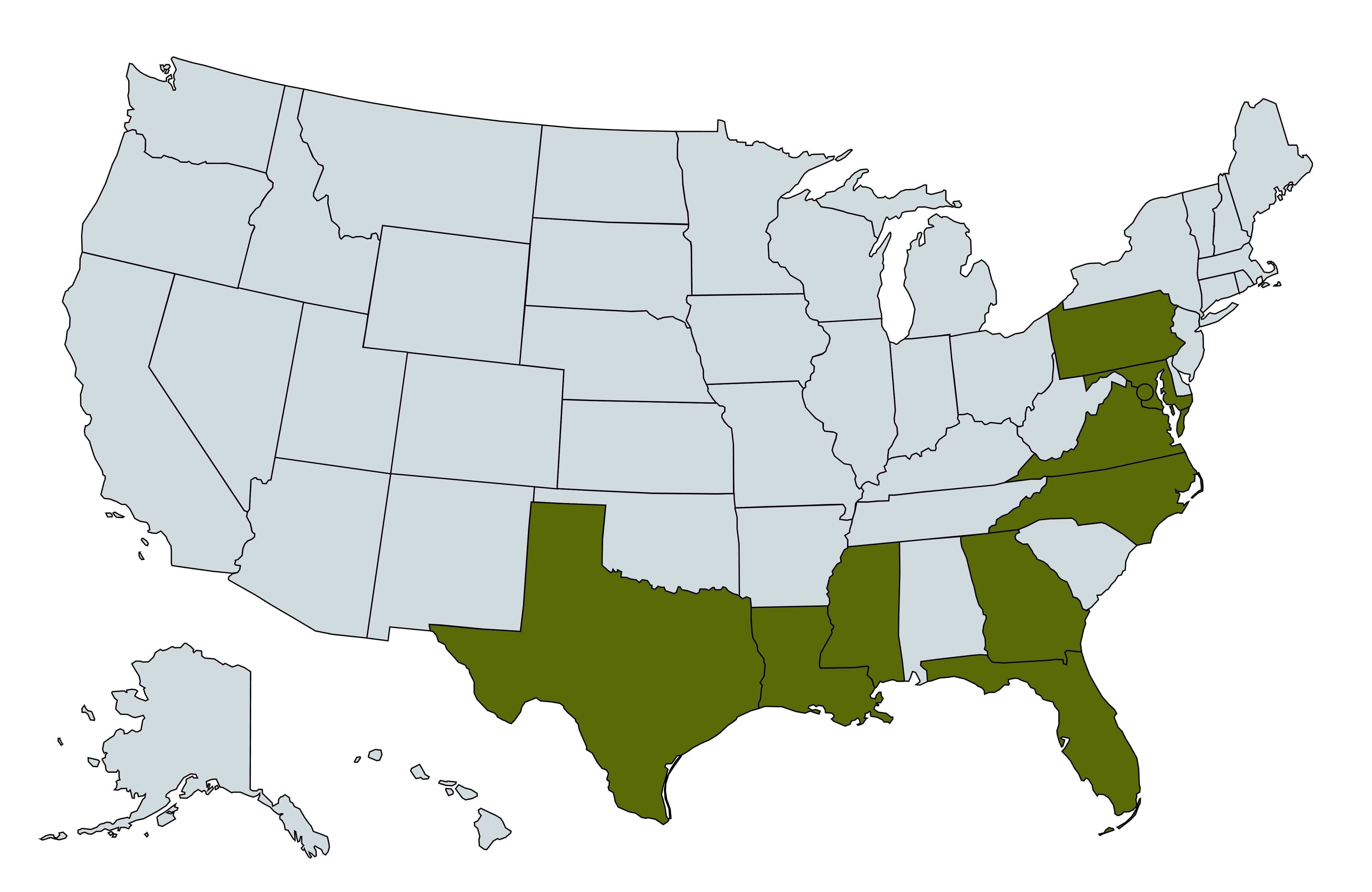
Credit: Abdullah A. Alomar, UF/IFAS
Keystone Virus Transmission Cycle
The transmission cycle of KEYV is poorly understood. It is proposed to be a zoonotic pathogen (a pathogen that circulates among non-human vertebrate animals) that occasionally infects humans. It has been suggested that KEYV can be maintained in a sylvan zoonotic transmission cycle involving vertebrate wildlife species (possibly eastern gray squirrels and raccoons), which may serve as virus-amplifying hosts (animal species that are susceptible to infection and serve as a source of infection for new mosquito vectors), and mosquito vectors (Figure 2) (LeDuc et al. 1975b). Aedes atlanticus (Figure 3) is suspected to be the primary KEYV vector (Sudia et al. 1971).
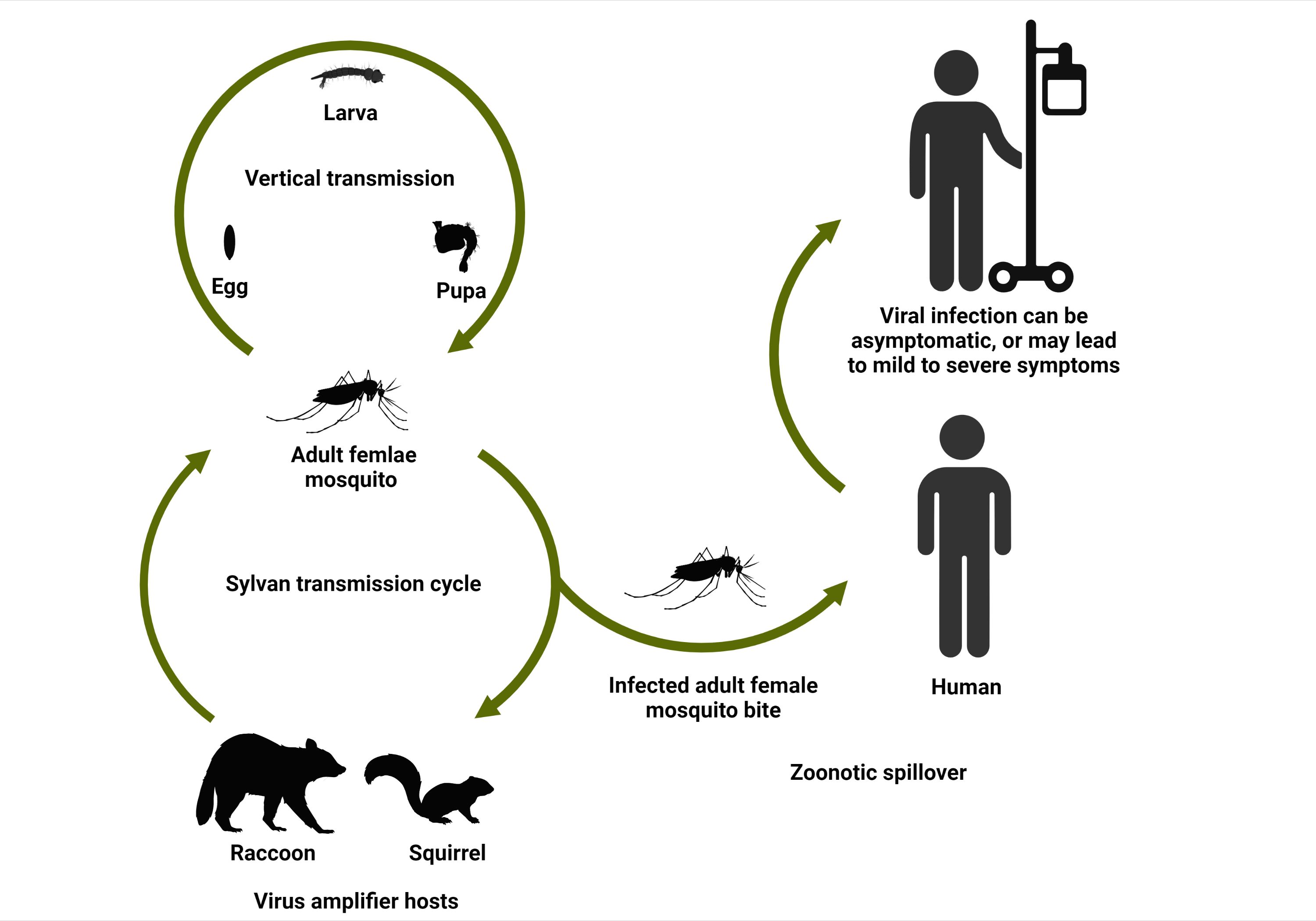
Credit: Abdullah A. Alomar, UF/IFAS

Credit: Lawrence E. Reeves, UF/IFAS
Keystone virus has also been found in field-collected Ae. canadensis, Ae. infirmatus, Ae. taeniorhynchus, Ae. triseriatus, Ae. vexans, Anopheles crucians complex species, An. punctipennis, Cx. nigripalpus, and in an undetermined species of Culex subgenus Melanoconion. Of these, Ae. infirmatus and Ae. taeniorhynchus may also have a role in KEYV transmission (Sudia et al. 1971; Wills et al. 1974). Detections of KEYV in mosquito species that are not able to transmit the virus to new vertebrate hosts can occur when a mosquito feeds from an infected vertebrate, but such detections do not necessarily imply that the mosquito species is a capable vector for KEYV. It is important to note that Ae. atlanticus is morphologically similar to Ae. tormentor, and the two species co-occur in much of the southeastern United States, making discrimination of potential vectors challenging. Both species are present throughout the southeastern coastal plain but absent from the Appalachian Mountains, north to southern New England, and west to eastern Texas and Oklahoma (Darsie and Morris 2003). In Florida, Ae. atlanticus occurs throughout the state, while Ae. tormentor is absent from the southern peninsula. Some early studies that identified KEYV in mosquitoes did not distinguish between Ae. atlanticus and Ae. tormentor (e.g., Sudia et al. 1971). Reliable morphological characters distinguishing the two species (Figure 4) were not reported until 1979 (Roberts and Scanlon 1979), so the role of Ae. tormentor as a potential vector for KEYV is nebulous. It should be further noted that a recently introduced Caribbean mosquito species, Ae. pertinax, closely resembles both Ae. atlanticus and Ae. tormentor, and so future research should account for the presence of this species, which now occurs in Florida in Collier, Indian River, Lee, Miami-Dade, and Sarasota Counties, and likely others (Shroyer et al. 2015; Burkett-Cadena et al. 2019; Riles and Connelly 2020; Tyler-Julian et al. 2022).

Credit: Lawrence E. Reeves, UF/IFAS
Keystone virus, or serological evidence of exposure to KEYV, has been found in white-tailed deer, domestic goats, raccoons, eastern gray squirrels, hispid cotton rats, and eastern cottontail rabbits (Taylor et al. 1971; Watts et al. 1982), but the importance of these animals in the KEYV transmission cycle is unknown. White-tailed deer are likely dead-end hosts (hosts that can become infected, but rarely infect new mosquito vectors because of insufficient virus circulating in the blood) for KEYV (Issel et al. 1973). The virus has been observed to be vertically transmitted (transmitted from female mosquitoes to their progeny) in Ae. atlanticus, a potential mechanism that could allow the virus to be maintained in nature during unfavorable environmental conditions (LeDuc et al. 1975b). Keystone virus has been most frequently detected in mosquitoes during late summer, with a peak in August (Sudia et al. 1971). Since there have been limited studies on the KEYV transmission cycle, understanding factors that may contribute to maintaining the virus in the ecosystem and to spillover of KEYV to humans is essential to prevent the potential emergence and spread of the virus.
Clinical Manifestations of Keystone Virus Infection in Humans
Viral infections of humans with California serogroup viruses have been documented to cause signs and symptoms that range from mild (a rash or fever) to severe (acute neurological disease) (Evans and Peterson 2019). Previous cases of humans infection with KEYV have been asymptomatic (did not develop symptoms) or produced mild symptoms, including fatigue, fever, ankle discomfort, headache, vomiting, and joint pain. However, very few human cases have been identified or have had clinical signs described. A clinical manifestation of erythematous papular rash (a skin rash caused by damaged blood vessels) was observed to be associated with a recent human KEYV infection (Lednicky et al. 2018). It might be possible, in rare cases, that KEYV infection may lead to encephalitis (brain inflammation) as observed with other California serogroup viruses, considering that KEYV is a member of this serogroup of viruses (Evans et al. 2019). Few data are available that are informative about the frequency of KEYV infections in humans that lead to severe disease, but low frequencies of human infections with other related mosquito-vectored viruses, those of the former California serogroup of arboviruses, are known to lead to encephalitis or meningitis. It is possible that KEYV represents an underappreciated cause of encephalitis or meningitis in Florida, but additional research is needed to fully understand the risks and burden KEYV imposes on public health (Henry et al. 2022).
Keystone Virus Diagnosis
An accurate diagnostic test for KEYV infection is essential and important because KEYV infection in humans shares similar symptoms with other more common arboviral pathogen infections, such as dengue virus. A doctor or other healthcare provider may ask the patient about their travel history and may order blood, saliva, or urine samples for molecular tests to determine whether or not the patient is infected with KEYV. Common methods for diagnosing current KEYV infection (or previous infection as indicated by serological detection of antibodies to KEYV) include viral isolation (Lednicky et al. 2018), direct detection of virions by electron microscopy, serological identification of virus or antigen, and detection of viral genomes by reverse transcription polymerase chain reaction.
Keystone Virus Treatment and Prevention
Since there is no specific vaccine or antiviral treatment for clinical KEYV infection, clinical care is supportive. The primary mean of preventing KEYV infection is avoidance of mosquito bites. Several personal protective measures can lower the potential of getting bitten. These include the use of protective clothing and mosquito repellents as well as staying indoors during times of high mosquito abundance or activity and avoiding places where mosquitoes are active. Aedes atlanticus mosquitoes, the suspected primary vectors of KEYV, bite during the day (Carpenter and LaCasse 1955) and may also feed at night. It is highly recommended to use EPA-registered mosquito repellent products (e.g., DEET, Picaridin, IR3535, oil of lemon eucalyptus, and 2-undecanone) that can provide long-lasting protection against the bites of mosquitoes. Whether KEYV can be transmitted by means other than mosquito bites, such as through sex, blood transfusion, or mother to fetus as observed with certain other arboviral pathogens is yet to be determined. Further study is needed to improve our understanding of KEYV distribution and occurrence, signs and symptoms, modes of transmission, diagnosis, and treatment in humans.
References
Bond, J. O., W. M. Hammon, A. L. Lewis, G. E. Sather, and D. J. Taylor. 1966. “California Group Arboviruses in Florida and Report of a New Strain, Keystone Virus.” Public Health Reports 81:607. https://doi.org/10.2307/4592788
Burkett-Cadena, N. D., I. Hoyer, E. Blosser, and L. E. Reeves. 2019. “Human-Powered Pop-Up Resting Shelter for Sampling Cavity-Resting Mosquitoes.” Acta Tropica 190:288-292. https://doi.org/10.1016/j.actatropica.2018.12.002
Carpenter, S. J., and W. J. LaCasse. 1955. Mosquitoes of North America (North of Mexico). University of California Press, Berkeley and Los Angeles. https://doi.org/10.1525/9780520325098
Darsie, R. F., and C. D. Morris. 2003. “Keys to the Adult Females and Fourth Instar Larvae of the Mosquitoes of Florida (Diptera: Culicidae).” Technical Bulletin of the Florida Mosquito Control Association.
Evans, A. B., and K. E. Peterson. 2019. “Throw out the Map: Neuropathogenesis of the Globally Expanding California Serogroup of Orthobunyaviruses.” Viruses 11:794. https://doi.org/10.3390/v11090794
Evans, A. B., C. W. Winkler, and K. E. Peterson. 2019. “Differences in Neuropathogenesis of Encephalitic California Serogroup Viruses.” Emerging Infectious Diseases 25:728. https://doi.org/10.3201/eid2504.181016
Fine, P. E., and J. W. LeDuc. 1978. “Towards a Quantitative Understanding of the Epidemiology of Keystone Virus in the Eastern United States.” The American Journal of Tropical Medicine and Hygiene 27: 322-338. https://doi.org/10.4269/ajtmh.1978.27.322
Henry, C. J., A. N. Pillai, J. A. Lednicky, J. G. Morris Jr., and T. J. Hladish. 2022. “Ecology and Public Health Burden of Keystone Virus in Florida.” Epidemics 39:100555. https://doi.org/10.1016/j.epidem.2022.100555
Issel, C. J., G. L. Hoff, and D. O. Trainer. 1973. “Serological Evidence of Infection of White-Tailed Deer in Texas with Three California Group Arboviruses (Jamestown Canyon, San Angelo, Keystone).” Journal of Wildlife Diseases 9:245-248. https://doi.org/10.7589/0090-3558-9.3.245
LeDuc, J. W., W. Suyemoto, T. J. Keefe, J. F. Burger, B. F. Eldridge, and P. K. Russell. 1975a. “Ecology of California Encephalitis Viruses on the Del Mar Va Peninsula. I. Virus Isolations from Mosquitoes.” The American Journal of Tropical Medicine and Hygiene 24: 118-123. https://doi.org/10.4269/ajtmh.1975.24.118
LeDuc, J. W., J. F. Burger, B. F. Eldridge, and P. K. Russell. 1975b. “Ecology of Keystone Virus, a Transovarially Maintained Arbovirus.” Annals of the New York Academy of Science, 266:144-51. https://doi.org/10.1111/j.1749-6632.1975.tb35095.x
Lednicky, J. A., S. K. White, C. J. Stephenson, K. Cherabuddi, J. C. Loeb, N. Moussatche, A. Lednicky, and J. G. Morris Jr. 2019. “Keystone Virus Isolated from a Florida Teenager with Rash and Subjective Fever: Another Endemic Arbovirus in the Southeastern United States?” Clinical Infectious Diseases 68:143-145. https://doi.org/10.1093/cid/ciy485
Parkin, W. E., W. M. Hammon, and G. E. Sather. 1972. “Review of Current Epidemiological Literature on viruses of the California Arbovirus Group.” American Journal of Tropical Medicine and Hygiene 21:964-978.
Plowright, R. K., C. R. Parrish, H. McCallum, P. J. Hudson, A. I. Ko, A. L. Graham, and J.O. Lloyd-Smith. 2017. “Pathways to Zoonotic Spillover.” Nature Reviews Microbiology 15:502-510. https://doi.org/10.1038/nrmicro.2017.45
Riles, M. T., and C. R. Connelly. 2020. “An Update of the Mosquito Records of Florida Counties, USA.” Journal of the American Mosquito Control Association 36:107–111. https://doi.org/10.2987/20-6923.1
Roberts, D. R., and J. E. Scanlon. 1979. “An Evaluation of Morphological Characters for Separating Females of Aedes (Ochlerotatus) atlanticus Dyar and Knab and Aedes (Ochlerotatus) tormentor Dyar and Knab (Diptera: Culicidae).” Mosquito Systematics 11: 203-208.
Shroyer, D. A., B. A. Harrison, B. J. Bintz, M. R. Wilson, C. B. Sither, and B. D. Byrd. 2015. “Aedes pertinax, a Newly Recognized Mosquito Species in the United States.” Journal of the American Mosquito Control Association 31:97-100. https://doi.org/10.2987/14-6447R.1
Sudia, W.D., V. F. Newhouse, C. H. Calisher, and R. W. Chamberlain. 1971. “California Group Arboviruses: Isolations from Mosquitoes in North America.” Mosquito News 31:576-600.
Taylor, D. J., A. L. Lewis, J. D. Edman, W. L. Jennings. 1971. “California Group Arboviruses in Florida.” American Journal of Tropical Medicine and Hygiene 20:139-145. https://doi.org/10.4269/ajtmh.1971.20.139
Tyler-Julian, K., L. E. Reeves, A. Lloyd, and D. Hoel. 2022. “Aedes pertinax and Culex interrogator: Two Mosquito Species New to Lee County, Florida.” Journal of the Florida Mosquito Control Association 69:16-20. https://doi.org/10.32473/jfmca.v69i1.130621
Watts, D. M., C. L. Bailey, N. T. Roberts, R. F. Tammariello, J. M. Dalrymple, and G. C. Clark. 1988. “Maintenance and Transmission Of Keystone Virus by Aedes atlanticus (Diptera: Culicidae) and the Gray Squirrel in the Pocomoke Cypress Swamp, Maryland.” Journal of Medical Entomology 25:493-500. https://doi.org/10.1093/jmedent/25.6.493
Watts, D.M., J. W. LeDuc, C. L. Bailey, J. M. Dalrymple, and T. P. Gargan. 1982. “Serologic Evidence of Jamestown Canyon and Keystone Virus Infection in Vertebrates of the DelMarVa Peninsula.” American Journal of Tropical Medicine and Hygiene 31:1245-1251. https://doi.org/10.4269/ajtmh.1982.31.1245
Wills, W., V. Pidcoe, D. F. Carroll, and J. E. Satz. 1974. “Isolation of California Group Arboviruses from Pennsylvania Mosquitoes – 1971, 1972.” Mosquito News 34:376–381.