Summary
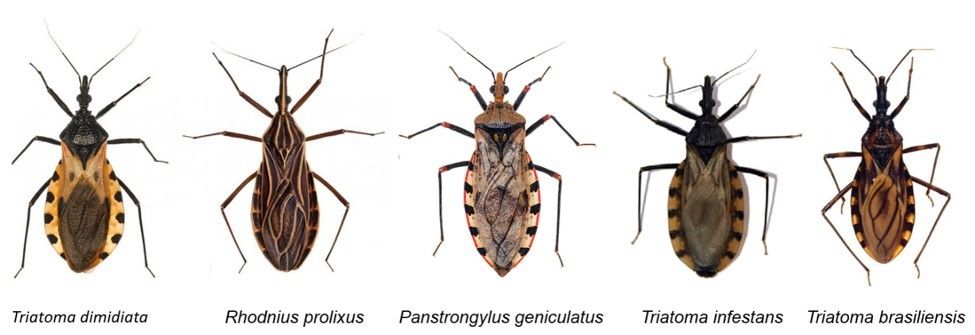
Chagas disease, also known as American trypanosomiasis, is a potentially fatal, chronic disease caused by infection with Trypanosoma cruzi, a protozoan parasite. Chagas disease is recognized by the World Health Organization as one of the most neglected tropical diseases with 6–8 million cases and 50,000 resulting deaths per year (Rassi et al. 2010; WHO 2022). Humans and animals usually become infected with T. cruzi after being bitten by triatomine bugs, T. cruzi insect vectors that are often referred to as kissing or conenose bugs (Figure 1). Triatomine bugs infected with T. cruzi are only found in the Americas. Chagas disease cases caused by triatomine bug transmission generally occur in Mexico, Central America, and South America. However, this disease is becoming more recognized in non-endemic areas, such as the United States, due to the migration of asymptomatic infected people to these areas (CDC 2022a). Currently, approximately 300,000 people are infected with Chagas disease in the United States, with 18,000 of these individuals in Florida (Bern and Montgomery 2009; Levesque 2021; Irish et al. 2022). Additionally, approximately 75 suspected or confirmed human Chagas disease cases resulting from local transmission via triatomines were documented in the United States from 2000 to 2018 (Lynn et al. 2020). However, more research is needed to understand the true risk of transmission in areas of the United States where triatomines are present. Awareness of Chagas disease and its vectors, transmission routes, symptoms, diagnoses, and treatments is critical in endemic and non-endemic areas of the world to ensure that Chagas disease cases are detected and treated early (Ramsey and Schofield 2003; Forsyth et al. 2022). This publication is intended for anyone interested in learning more about vector-borne transmission of T. cruzi and Chagas disease.
Credit: Centers for Disease Prevention and Control (CDC) Southeastern Center of Excellence in Vector-Borne Diseases
Chagas Disease (American Trypanosomiasis)
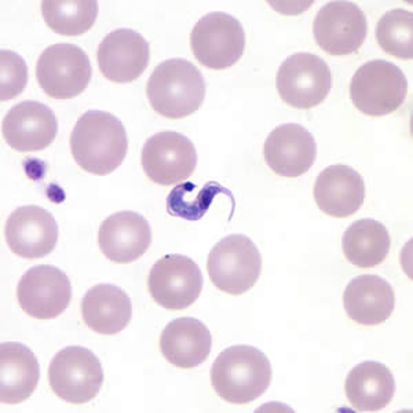
Chagas disease, also known as American trypanosomiasis, is caused by the protozoan parasite Trypanosoma cruzi (Figure 2). The disease is named after Carlos Chagas, the Brazilian physician who discovered the disease in 1909. Though not identified until 1909, Chagas is an ancient disease that has afflicted humans for thousands of years. It is believed that T. cruzi was introduced to South America approximately 7–10 million years ago by bats (Steverding 2014). The oldest known human case of Chagas disease occurred 9,000 years ago as evidenced by T. cruzi found in desiccated human mummies in South America (Aufderheide et al. 2004). Humans and animals usually become infected by T. cruzi through interactions with triatomine bugs, which are often referred to as kissing or conenose bugs. Triatomine bugs that carry T. cruzi are only found in the Americas, and Chagas disease cases due to T. cruzi triatomine bug transmission generally occur in rural areas of Mexico, Central America, and South America. While Chagas disease was once considered a disease that plagued only Latin America, its recognition in countries that are considered non-endemic has led to an increase in number of identified disease cases in recent years. The migration of chronically infected people from endemic countries to other areas of the world has allowed the worldwide distribution of Chagas disease cases to expand (Antinori et al. 2017) (Figure 3). Across the world, approximately 65–75 million people are at risk of contracting Chagas disease (Chao et al. 2020).
Credit: CDC
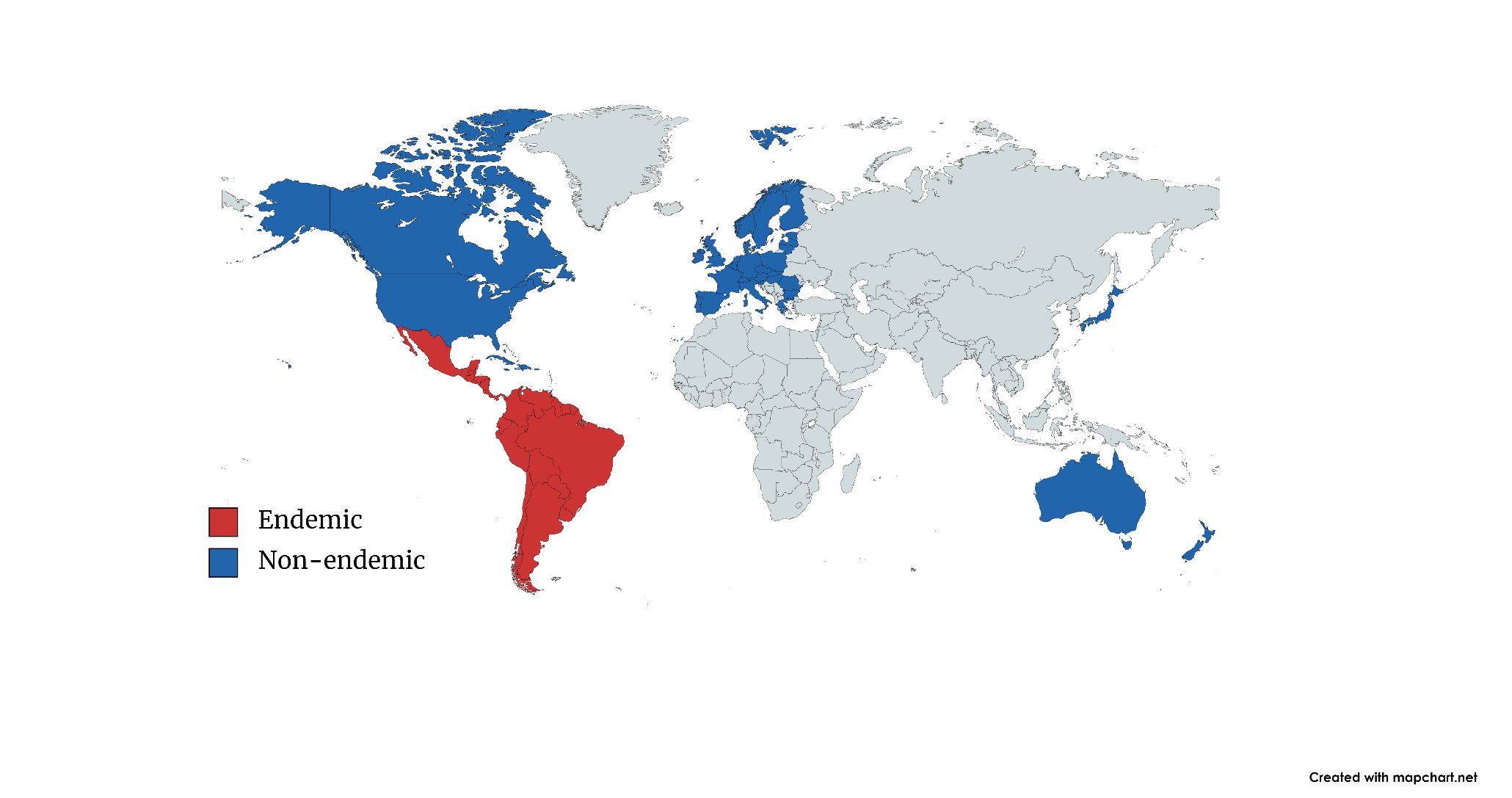
Credit: Map created using mapchart.net by Eva A. Buckner, UF/IFAS
Trypanosoma cruzi Transmission
In endemic countries, T. cruzi infections occur primarily through contact with feces or urine of infected blood-sucking triatomine bugs. These bugs are active at night and hide during the day in cracks and crevices in the walls and roofs of homes and nearby structures like chicken coops, animal pens, and storage buildings. At night, they emerge from their hiding spots and seek animals on which to blood feed. Sleeping humans make easy targets, and triatomine bugs usually bite exposed areas of the skin, such as on the face near the mouth or nose, giving them their common name of kissing bugs. During or soon after blood-feeding, some triatomine bugs defecate or urinate close to the bite site. The feces or urine of an infected triatomine bug may get rubbed into the site of the bite, other skin breaks, the eyes, or the mouth, leading to T. cruzi entering the person’s bloodstream. Many wild mammals, such as armadillos, opossums, raccoons, and small rodents like wood rats have been identified as T. cruzi reservoir hosts (CDC 2021). Additionally, domesticated mammals like dogs, cats, and guinea pigs can serve as T. cruzi reservoir hosts (Gürtler and Cardinal 2015). Though T. cruzi infections in humans generally occur through vector-borne transmission by triatomine bugs, other routes of T. cruzi transmission exist, including consumption of T. cruzi contaminated food and/or drink, congenital transmission from infected mother to fetus, blood transfusions, organ transplantations, and laboratory accidents (Liu and Zhou 2015).
Triatomine Bugs, Vectors of Trypanosoma cruzi
Triatomine bugs (Hemiptera, Reduviidae, Triatominae) are insects that are known by many different names, such as reduviid bugs, kissing bugs, vampire bugs, and conenose bugs. These insects range between 0.75 and 1.75 inches in length with a cone-shaped head and a long, piercing proboscis, which is used for bloodsucking and is often referred to as a beak (Figure 4). While some characteristics vary by species, in general, adult triatomine bugs have brown or black flattened bodies with wide abdomens (Figure 1). The sides of the abdomen stick out beyond the wing margins and can be marked with red, orange, or yellow stripes (Figure 5). Several other bugs can be mistaken for triatomines including assassin bugs, wheel bugs, and leaf-footed bugs (CDC 2019a) (Figure 6). For moreon look-alike leaf-footed bugs, see https://entnemdept.ufl.edu/creatures/orn/leaffooted_bug.htm. Triatomine bugs have an incomplete life cycle that consists of eggs, five nymphal instars (immature stages that look like adults but lack fully developed wings), and adults. All nymphal instars require a bloodmeal to molt to the next stage, and both male and female adults blood-feed.

Credit: S. Kjos, CDC

Credit: (A) S. Kjos, CDC; (B) J. Gathany, CDC

Credit: A) and (C), P. J. Bryant, CDC; (B), B. Drees, CDC
Worldwide, there are at least 150 different triatomine bug species that are potential vectors of T. cruzi (de Oliveira et al. 2018). This parasite may spread from the triatomine bug insect vector to more than 150 different species of mammals on which they have been documented blood-feeding, including humans (Rassi et al. 2010). Triatomine bugs can be found indoors, within the cracks and holes of low-quality housing, or outdoors underneath porches, in rock piles, in woodpiles, or under tree bark. They are also likely to be found outdoors close to potential bloodmeal hosts in rodent nests, animal burrows, dog kennels, or chicken coops (CDC 2022b). For more information on triatomine bugs, please see the Featured Creatures publication on the eastern bloodsucking conenose available at https://entnemdept.ufl.edu/creatures/urban/triatoma_sanguisuga.htm.
Symptoms
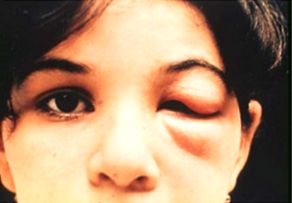
The symptoms of Chagas disease depend on its stage of infection, of which there are two: acute and chronic. The acute stage is the initial phase of infection during which T. cruzi can be detected in blood samples (Prata 2001). The acute stage can last for several weeks, and most cases will be asymptomatic. People who develop symptoms generally experience fever, headache, swollen lymph nodes, body aches, loss of appetite, diarrhea, and vomiting (Añez et al. 1999). One characteristic symptom of Chagas disease that may develop during the acute stage is a chagoma, a red, raised nodule that develops where T. cruzi has entered the body. When a chagoma occurs specifically near the eye, it is referred to as a Romaña’s sign (Ortiz-Medina and Pons 1974) (Figure 7). Severe symptoms like inflammation of the brain and/or heart are rare during the acute stage but can potentially lead to death in those who are immunocompromised (Lopez-Velez et al. 2019).
Credit: WHO/TDR Image Library
Following the acute stage, a person enters the chronic stage, during which few to no T. cruzi can be detected in blood samples (CDC 2019b). Within the chronic stage are the indeterminate or asymptomatic phase and the determinate or symptomatic phase. All chronic infections begin as indeterminate. Once in the indeterminate chronic phase, people can remain there for the rest of their lives. For approximately 20%–30% of those in the indeterminate chronic phase, however, their infections slowly progress into the determinate chronic phase over the span of months to decades (WHO 2002; Patel and Sethi 2009).
While it is still not understood what triggers the indeterminant phase to transition into determinate, symptoms of this phase are serious and primarily affect the heart, esophagus, and/or colon (Chatelain 2017; Lidani et al. 2019). Up to 40% of individuals with chronic Chagas disease are affected by Chagas heart disease, the principal cause of Chagas-related morbidity and mortality (Andrade et al. 2011). Patients with Chagas heart disease require medical intervention to help manage cardiac complications. Depending on the severity of a patient’s Chagas heart disease, a heart transplant could be needed (Biolo et al. 2010). Sudden cardiac arrest, stroke, and progressive heart failure can lead to death in those with Chagas heart disease (Coura 2007; Lima-Costa et al. 2010). Fortunately, chronic Chagas disease symptoms that affect organs other than the heart, such as the esophagus and colon, are not as common or as severe as those impacting the heart. However, chronic Chagas disease symptoms involving the esophagus and/or colon may impact a person’s ability to eat or to pass stool (CDC 2019b).
Diagnosis
Because many people with acute Chagas disease do not exhibit any symptoms and those that do may only display nonspecific symptoms, diagnosis based on symptom evaluation alone may not be feasible. Certainly, recent travel to an endemic country coupled with the presentation of related symptoms can direct clinicians to test for Chagas disease. Several types of blood tests can be performed to either confirm the presence of the T. cruzi parasite or to detect the presence of antibodies to T. cruzi.
The laboratory methods used to diagnose Chagas disease depend on the patient’s stage of infection. During its acute stage when many parasites are present in the blood, Chagas disease can be diagnosed by using light microscopy to visualize T. cruzi in prepared blood smear slides. Additionally, during the acute stage, polymerase chain reaction (PCR) can be used to detect the presence of T. cruzi DNA in tissue samples but is not a proven method for the diagnosis of chronic Chagas disease (Brasil et al. 2010; Luquetti and Schmunis 2017).
Serological tests detect antibodies against the T. cruzi parasite in the blood and are the primary modality for a Chagas disease diagnosis during both acute and chronic stages of infection currently. There are three serological tests conventionally used to diagnose Chagas in its chronic stage: indirect hemagglutination (IHA), indirect immunofluorescence (IIF), and enzyme-linked immunosorbent assay (ELISA). All three serological tests have limitations and lack both sensitivity and specificity; therefore, the World Health Organization and the US Chagas Disease Diagnostic Working Group recommend performing at least two separate tests, which are a different methodology or T. cruzi antigen preparation, for a conclusive diagnosis of Chagas disease (Forsyth et al. 2022). However, one positive ELISA test is enough to remove a blood sample from a blood bank (WHO 2015).
Treatment
Chagas disease can be cured during its acute stage using antiparasitic drugs, such as benznidazole and nifurtimox. Once the disease reaches its chronic stage, most treatments focus on helping patients manage their chronic Chagas disease symptoms (Apt 2017). Antiparasitic drug treatment may be continued during the chronic stage to reduce the severity of some disease symptoms (Morillo et al. 2015). New treatments are greatly needed for Chagas disease, because the antiparasitic drugs currently in use may cause adverse reactions in some patients and drug resistance in T. cruzi (Filardi and Brener 1987; Garcia-Huertas and Cardona-Castro 2021). Disclaimer: The information in this and the previous section is provided for informational purposes only. It is not meant to substitute for professional medical diagnosis or treatment.
Chagas Disease in the United States
All components required for T. cruzi transmission by triatomine bugs to humans are present in United States. For example, 11 triatomine bug species have been documented in the United States. Though the distribution of each species varies, triatomine bugs are found in the southern two-thirds of states (CDC 2019b) (Figure 8). Also, within the United States, more than two dozen wildlife species, including raccoons, opossums, armadillos, foxes, mice, squirrels, coyotes, skunks, bats, deer, wood rats, and non-human primates in captivity have been found infected with T. cruzi (Bern et al. 2011; Hodo et al. 2016; Gunter et al. 2018; Busselman and Hamer 2022). Trypanosoma cruzi infections have been detected in the majority of all triatomine bug species present in the United States. (Bern et al. 2011; Zeledon et al. 2012). Lastly, blood meals taken from humans have been recorded in triatomine bugs collected from the United States (Klotz et al. 2014; Beatty et al. 2019).
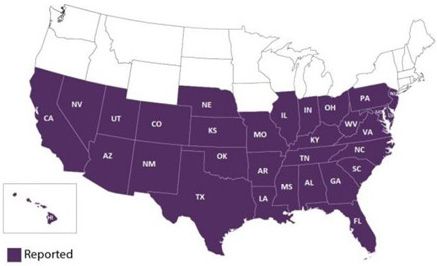
Credit: CDC
While the number of people living with Chagas disease in the United States is unknown, approximately 300,000 are estimated to have become infected with T. cruzi in endemic regions of Latin America prior to immigration to the United States (Bern and Montgomery 2009; Irish et al. 2022). Even though all the elements detailed in the paragraph above align to allow for triatomine bug transmission of T. cruzi to humans in the United States, Chagas disease cases due to triatomine bug transmission are not well documented (Webber et al. 2017; Dodd et al. 2019). According to the CDC, the likelihood of acquiring a T. cruzi infection from a triatomine bug in the United States is low, even if the bug is infected (CDC 2019b). Many factors are thought to be responsible for the low number of locally acquired T. cruzi infections documented in the United States, including behavior differences in triatomine bug species native to the United States compared to those native to Mexico, Central and South America. Triatomine bug species native to the United States seem to wait longer to defecate after blood-feeding compared to those triatomine bug species known to be the vectors responsible for the majority of T. cruzi infections in South America. A longer period of time between blood-feeding and defecating may make triatomine bug species native to the United States less likely to defecate while still on a host, preventing transmission of T. cruzi to that host (Bern and Montgomery 2009).
Another possible reason for fewer locally acquired Chagas disease cases in the United States is well-constructed homes with window screens and other barriers that prevent triatomine bugs from invading homes (Bern and Montgomery 2009). Triatomine bugs are primarily found in or near forested areas within the southern United States. As a result, human-triatomine bug contact is likely to occur only in this type of habitat. However, triatomine bugs may be found close to homes near wooded areas with dog kennels, livestock, chicken coops and/or woodpiles. Because triatomine bugs are attracted to these bloodmeal sources and hiding places, the likelihood of a human encountering a triatomine bug near a home in this type of environment is increased, as is the chance that a triatomine bug could make its way inside the home (Wisely and Beatty 2021).
While transmission in the United States appears to be low with less than 100 suspected or confirmed vector-borne, locally acquired Chagas disease cases recorded from 2000 to 2018 (Lynn et al. 2020), more research is needed to uncover true transmission dynamics. To better assess local transmission rates in the United States, Lynn et al. (2020) recommend making Chagas disease a mandatory reportable condition in all southern states instead of just the current seven states of Texas, Arizona, Arkansas, Tennessee, Mississippi, Louisiana, and Utah where it is mandatory. Increasing triatomine bug surveillance and serosurveys for Trypanosoma cruzi antibodies in southern states are also recommended (Lynn et al. 2020).
Chagas Disease in Florida
Two native triatomine bug species, Triatoma sanguisuga and Paratriatoma lecticularia, and one invasive triatomine bug species, Triatoma rubrofasciata, all vectors of T. cruzi, are present in Florida (Figure 9). Triatoma sanguisuga (Figure 10) is the most common triatomine bug species in the state and found in over half of Florida’s counties. Like triatomine bugs elsewhere in the southern United States, Florida’s triatomine bugs live near or in wooded areas. People who spend time in this type of habitat or live in homes near wooded areas with bloodmeal sources (e.g., chicken coops, dog kennels, and livestock) and hiding places (e.g., woodpile) available nearby are more likely to encounter triatomine bugs (Wisely and Beatty 2021). However, the risk of triatomine bug-transmitted T. cruzi infection in Florida is low, and no locally acquired Chagas disease cases have been documented in the state (FDOH 2021).
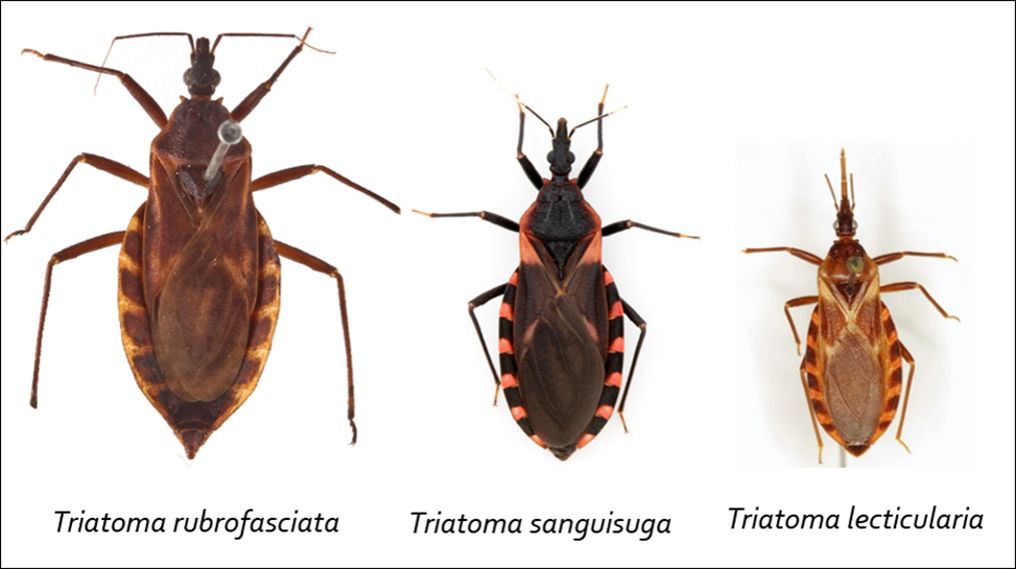
Credit: CDC Southeastern Center of Excellence in Vector-Borne Diseases

Credit: Dr. Norman Beatty, University of Florida
Researchers estimate that 18,000 people who became infected with T. cruzi while living in endemic regions of Latin America before immigrating to the United States are estimated to be living with Chagas disease in Florida (Beatty and Klotz 2020). The actual number of people with Chagas disease in Florida is most likely higher than 18,000 due to lack of awareness and diagnosis. Therefore, Dr. Norman Beatty at the University of Florida is leading an effort to determine the prevalence of Chagas disease among Latin Americans in Florida as well as those exposed to native kissing bugs (Levesque 2021). Additionally, to better understand T. cruzi distribution and which triatomine bug species could serve as vectors to humans in Florida, Beatty and colleagues are testing triatomine bugs captured from throughout the state for T. cruzi and performing blood meal analysis on them. Nearly 30% of the triatomine bugs collected from 2013 to 2022were positive for T. cruzi. Blood meal analysis revealed that these insects had fed on humans, amphibians, reptiles, opossums, and even cockroaches (Beatty et al. 2022). More information about this research can be found at https://id.medicine.ufl.edu/research/bench-research/beatty-lab-2/.
Prevention and Control
Because there is no vaccine available to prevent Chagas disease, a multifaceted approach of controlling triatomine bugs, screening for disease, and educating the public is needed to reduce exposure to triatomine bugs and prevent disease cases. Recommendations for preventing the entrance of triatomine bugs into a house include the following:
- Using screens on windows and doors.
- Turning off outside lights at night.
- Sealing all gaps and cracks in the walls and foundation of the house.
- Not stacking firewood against the house.
- Eliminating debris around the house.
- Installing weather stripping around doors and windows.
- Having pets sleep indoors.
- Keeping chicken coops, dog kennels, and livestock away from the house (Brown 2015; CDC 2022b; Wisely and Beatty 2021).
Targeted screening of individuals at risk, newborns, and blood and organ donors must be conducted to reduce the spread of Chagas disease. Screening high-risk individuals would also allow for early detection of Chagas disease when treatment is most critical (CDC 2022a). Additionally, education to increase awareness about Chagas disease is needed not just in areas where the disease is endemic but also in non-endemic areas where Latin Americans potentially infected with T. cruzi have immigrated. Increased awareness of Chagas disease among those at risk and their physicians will hopefully lead to early detection and treatment before severe symptoms of chronic Chagas are experienced (Bern et al. 2020).
References
Andrade, J. P. D., J. A. Marin Neto, A. A. V. D. Paola, F. Vilas-Boas, G. M. M. Oliveira, F. Bacal, E. A. Bocchi, et al. 2011. "Latin American Guidelines for the Diagnosis and Treatment of Chagas' Heart Disease: Executive Summary." Arquivos brasileiros de cardiologia 96:434–442. https://doi.org/10.1590/S0066-782X2011000600002
Añez, N., H. Carrasco, H. Parada, G. Crisante, A. Rojas, N. Gonzalez, J.L. Ramirez, et al. 1999. "Acute Chagas' Disease in Western Venezuela: A Clinical, Seroparasitologic, and Epidemiologic Study." American Journal of Tropical Medicine & Hygiene 60 (2): 215–222. https://doi.org/10.4269/ajtmh.1999.60.215
Antinori, S., L. Galimberti, R. Bianco, R. Grande, M. Galli, and M. Corbellino. 2017. "Chagas Disease in Europe: A Review for the Internist in the Globalized World." European Journal of Internal Medicine 43:6–15. https://doi.org/10.1016/j.ejim.2017.05.001
Apt, W. 2017. “Treatment of Chagas Disease.” American Trypanosomiasis Chagas Disease: One Hundred Years of Research: Second Edition. Elsevier Inc. https://doi.org/10.1016/B978-0-12-801029-7.00032-0
Aufderheide, A.C., W. Salo, M. Madden, J. Streitz, J. Buikstra, F. Guhl, B. Arriaza, et al. 2004. "A 9,000-Year Record of Chagas' Disease." Proceedings of the National Academy of Sciences 101 (7): 2034–2039. https://doi.org/10.1073/pnas.0307312101
Beatty, N. L., N. Behrens-Bradley, M. Love, F. McCants, S. Smith, J. O. Schmidt, S. A. Hamer, P. L. Dorn, N. Ahmad, and S. A. Klotz. 2019. "Rapid Detection of Human Blood in Triatomines (Kissing Bugs) Utilizing a Lateral Flow Immunochromatographic Assay—A Pilot Study." Memorias do Instituto Oswaldo Cruz 114: e190047. https://doi.org/10.1590/0074-02760190047
Beatty, N. L., and S. A. Klotz. 2018. "The Midnight Bite! A Kissing Bug Nightmare.” American Journal of Medicine 131 (2): e43–e44. https://doi.org/10.1016/j.amjmed.2017.10.013
Beatty, N. L., and S. A. Klotz. 2020. “Autochthonous Chagas Disease in the United States: How Are People Getting Infected?” The American Journal of Tropical Medicine and Hygiene 103 (3): 967–969. https://doi.org/10.4269/ajtmh.19-0733
Beatty, N.L., C.R. Bhosale, Z.S. White, C. Torhorst, K.N. Wilson, R. Dorleans, T. Stenn, et al. 2022. “Florida Kissing Bugs (Hemiptera, Reduviidae, Triatominae) Invade Homes, Feed on Diverse Hosts, and Harbor Trypanosoma cruzi.” American Society of Tropical Medicine and Hygiene 71st Annual Scientific Meeting Poster. https://doi.org/10.13140/RG.2.2.29155.63528.
Bern, C., L. A. Messenger, J. D. Whitman, and J. H. Maguire. 2020. "Chagas Disease in the United States: a Public Health Approach." Clinical Microbiology Reviews 33(1). https://doi.org/10.1128/CMR.00023-19
Bern, C., S. Kjos, M. Yabsley, and S. P. Montgomery. 2011. "Trypanosoma cruzi and Chagas Disease in the United States." Clinical Microbiology Reviews 24:655–681. https://doi.org/10.1128/CMR.00005-11
Bern, C., and S. P. Montgomery. 2009. "An Estimate of the Burden of Chagas Disease in the United States." Clinical Infectious Diseases 49:e52–4. https://doi.org/10.1086/605091
Biolo, A., A. L. Ribeiro, and N. Clausell. 2010. "Chagas cardiomyopathy-where do we stand after a hundred years?" Progress in Cardiovascular Diseases 52 (4): 300–316. https://doi.org/10.1016/j.pcad.2009.11.008
Brasil, P. E., L. De Castro, A. M. Hasslocher-Moreno, L. H. Sangenis, and J. U. Braga. 2010. "ELISA versus PCR for Diagnosis of Chronic Chagas Disease: Systematic Review and Meta-Analysis." BMC Infectious Diseases 10:337. https://doi.org/10.1186/1471-2334-10-337
Brown, W. 2015. "Kissing Bugs and Chagas Disease." Texas A&M AgriLife Extension. November 25, 2015. Accessed October 18, 2021. https://schoolipm.tamu.edu/2015/11/25/school-pest-news-volume-15-issue-11-november-2015/
Busselman, R. E., and S. A. Hamer. 2022. "Chagas Disease Ecology in the United States: Recent Advances in Understanding Trypanosoma cruzi Transmission Among Triatomines, Wildlife, and Domestic Animals and a Quantitative Synthesis of Vector-Host Interactions." Annual Review of Animal Biosciences 10:325–348. https://doi.org/10.1146/annurev-animal-013120-043949
Centers for Disease Control and Prevention (CDC). 2022a. "About Chagas Disease." April 11, 2022. Accessed September 5, 2022. https://www.cdc.gov/parasites/chagas/gen_info/detailed.html#intro
Centers for Disease Control and Prevention (CDC). 2022b. "Triatomine Bug FAQs." April 13, 2022. Accessed September 5, 2022. https://www.cdc.gov/parasites/chagas/gen_info/vectors/index.html
Centers for Disease Control and Prevention (CDC). 2021. "American Trypanosomiasis." June 16, 2021. Accessed August 22, 2022. https://www.cdc.gov/dpdx/trypanosomiasisamerican/index.html
Centers for Disease Control and Prevention (CDC). 2019a. "Bugs Commonly Confused with Triatomine Bugs." July 17, 2019. Accessed September 9, 2022. https://www.cdc.gov/parasites/chagas/gen_info/vectors/confuse.html
Centers for Disease Control and Prevention (CDC). 2019b. "Prevention and Control". March 6, 2019. Accessed August 11, 2022. https://www.cdc.gov/parasites/chagas/prevent.html.
Chao, C., J. L. Leone, and C. A. Vigliano. 2020. "Chagas Disease: Historic Perspective." Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease 1866 (5): 1–15. https://doi.org/10.1016/j.bbadis.2020.165689
Chatelain, E. 2017. "Chagas disease research and development: Is there light at the end of the tunnel?" Computational and Structural Biotechnology Journal 15: 98–103. https://doi.org/10.1016/j.csbj.2016.12.002
Coura, J. R. 2007. "Chagas Disease: What Is Known and What Is Needed-A Background Article." Memórias do Instituto Oswaldo Cruz 102:113–122. https://doi.org/10.1590/S0074-02762007000900018
de Oliveira, A. B. B., K. C. C. Alevi, C. H. L. Imperador, F. F. Madeira, and M. T. V. Azeredo-Oliveira. 2018. "Parasite–Vector Interaction of Chagas Disease: A Mini-Review." American Journal of Tropical Medicine and Hygiene 98 (3): 653–655. https://doi.org/10.4269/ajtmh.17-0657
Dodd, R. Y., J. A. Groves, R. L. Townsend, E. P. Notari, G. A. Foster, B. Custer, M. P. Busch, and S. L. Stramer. 2019. "Impact of One‐Time Testing for Trypanosoma cruzi Antibodies among Blood Donors in the United States." Transfusion 59:1016–1023. https://doi.org/10.1111/trf.15118
Filardi, L. S., and Z. Brener. 1987. "Susceptibility and Natural Resistance of Trypanosoma cruzi Strains to Drugs Used Clinically in Chagas Disease." Transactions of the Royal Society of Tropical Medicine and Hygiene 81 (5): 755–759. https://doi.org/10.1016/0035-9203(87)90020-4
Forsyth, C. J., J. Manne-Goehler, C. Bern, J. Whitman, N. S. Hochberg, M. Edwards, R. Marcus, et al. 2022. "Recommendations for Screening and Diagnosis of Chagas Disease in the United States." The Journal of Infectious Diseases 225 (9): 1601–1610. https://doi.org/10.1093/infdis/jiab513
Garcia-Huertas, P., and N. Cardona-Castro. 2021. "Advances in the Treatment of Chagas Disease: Promising New Drugs, Plants and Targets." Biomedicine and Pharmacotherapy 142:112020. https://doi.org/10.1016/j.biopha.2021.112020
Gunter, S. M., C. Cordray, R. Gorchakov, I. Du, B. Dittmar, E. L. Brown, K. O. Murray, and M. S. Nolan. 2018. "Identification of White-Tailed Deer (Odocoileus virginianus) as a Novel Reservoir Species for Trypanosoma cruzi in Texas, USA." Journal of Wildlife Diseases 54:814–818. https://doi.org/10.7589/2017-09-223
Gurtler, R. E., and M. V. Cardinal. 2015. "Reservoir Host Competence and the Role of Domestic and Commensal Hosts in the Transmission of Trypanosoma cruzi." Acta Tropica 151:32–50. https://doi.org/10.1016/j.actatropica.2015.05.029
Hodo, C. L., C. C. Goodwin, B. C. Mayes, J. A. Mariscal, K. A. Waldrup, and S. A. Hamer. 2016. "Trypanosome Species, Including Trypanosoma cruzi, in Sylvatic and Peridomestic Bats of Texas, USA." Acta Tropica 164:259–266. https://doi.org/10.1016/j.actatropica.2016.09.013
Irish, A., J. D. Whitman, E. H. Clark, R. Marcus, and C. Bern. 2022. "Updated Estimates and Mapping for Prevalence of Chagas Disease among Adults, United States." Emerging Infectious Diseases 28 (7): 1313–1320. https://doi.org/10.3201/eid2807.212221
Klotz, S. A., J. O. Schmidt, P. L. Dorn, C. Ivanyi, K. R. Sullivan, and L. Stevens. 2014. "Free-Roaming Kissing Bugs, Vectors of Chagas Disease, Feed Often on Humans in the Southwest." The American Journal of Medicine 127:421–426. https://doi.org/10.1016/j.amjmed.2013.12.017
Levesque, B. 2021. "UF Health Scientists Study Prevalence of a Kissing Bug Disease among Latin Americans in Florida." University of Florida Health. February 8, 2021. Accessed August 12, 2022. https://ufhealth.org/news/2021/uf-health-scientists-study-prevalence-kissing-bug-disease-among-latin-americans-florida
Lidani, K. C. F., F. A. Andrade, L. Bavia, F. S. Damasceno, M. H. Beltrame, I. J. Messias-Reason, and T. L. Sandri. 2019. "Chagas Disease: From Discovery to a Worldwide Health Problem." Frontiers in Public Health 7:166. https://doi.org/10.3389/fpubh.2019.00166
Lima-Costa, M. F., D. L. Matos, and A. L. Ribeiro. 2010. "Chagas Disease Predicts 10-Year Stroke Mortality in Community-Dwelling Elderly: The Bambuí Cohort Study of Aging." Stroke 41:2477–2482. https://doi.org/10.1161/STROKEAHA.110.588061
Liu, Q., and X. N. Zhou. 2015. "Preventing the Transmission of American Trypanosomiasis and Its Spread into Non-Endemic Countries." Infectious Diseases of Poverty 4 (60): 1–11. https://doi.org/10.1186/s40249-015-0092-7
Lopez-Velez, R., F. F. Norman, and C. Bern. 2019. "American Trypanosomiasis (Chagas Disease)." In Hunter's Tropical Medicine and Emerging Infectious Disease, edited by E. T. Ryan, D. R. Hill, T. Solomon, T. P. Endy, and N. Aronson. 10th ed Elsevier; London, United Kingdom: pp. 762–765. https://doi.org/10.1016/B978-0-323-55512-8.00103-4
Luquetti, A. O., and G. A. Schmunis. 2017. “Diagnosis of Trypanosoma cruzi Infection.” p. 687-730. In American Trypanosomiasis Chagas Disease, edited by J. Telleira and M. Tibayrenc. Elsevier, Amsterdam, Netherlands. https://doi.org/10.1016/B978-0-12-801029-7.00030-7
Lynn, M. K., B. H. Bossak, P. A. Sandifer, A. Watson, and M. S. Nolan. 2020. “Contemporary Autochthonous Human Chagas Disease in the USA.” Acta Tropica 205:105361. https://doi.org/10.1016/j.actatropica.2020.105361
Montgomery, S. P., M. E. Parise, E. M. Dotson, and S. R. Bialek. 2016. "What do we know about chagas disease in the United States?" American Journal of Tropical Medicine and Hygiene 95:1225–1227. https://doi.org/10.4269/ajtmh.16-0213
Morillo, C. A., J. A. Marin-Neto, A. Avezum, S. Sosa-Estani, A. Rassi Jr., F. Rosas, E. Villena, R. Quiroz, R. Bonilla, C. Britto, and F. Guhl. 2015. "Randomized Trial of Benznidazole for Chronic Chagas' Cardiomyopathy." New England Journal of Medicine 373:1295–306. https://doi.org/10.1056/NEJMoa1507574
Ortiz-Medina, A., and S. Pons. 1974. "Manifestaciones cutáneas de la enfermedad de Chagas. Cuarta parte: Histopatología." Medicina Cutánea Ibero-Latino-Americana 2:391–397.
Patel, S., and A. Sethi. 2009. "Imported Tropical Diseases." Dermatological Therapy 22:538–549. https://doi.org/10.1111/j.1529-8019.2009.01275.x
Prata, A. 2001. "Clinical and Epidemiological Aspects of Chagas Disease." The Lancet Infectious Diseases 1:92–100. https://doi.org/10.1016/S1473-3099(01)00065-2
Ramsey, J. M., and C. J. Schofield. 2003. "Control of Chagas Disease Vectors." Salud Publica de Mexico 45 (2): 123–128. https://doi.org/10.1590/S0036-36342003000200010
Rassi Jr., A., A. Rassi, and J. A. Marin-Neto. 2010. "Chagas Disease." The Lancet 375 (9723): 1388–1402. https://doi.org/10.1016/S0140-6736(10)60061-X
Steverding, D. 2014. "The History of Chagas Disease." Parasites & Vectors 7 (317): 1–8. https://doi.org/10.1186/1756-3305-7-317
Webber, B. J., M. T. Pawlak, S. Valtier, C. C. Daniels, C. C. Tully, E. J. Wozniak, W. D. Roachell, F. X. Sanchez, A. A. Blasi, and T. L. Cropper. 2017. "Prevalence and Seroprevalence of Trypanosoma cruzi Infection in a Military Population in Texas." American Journal Tropical Medicine and Hygiene 97:1477–1481. https://doi.org/10.4269/ajtmh.17-0109
Wisely, S., and N. Beatty. 2021. "Chagas Disease—A Health Risk for Rural People in the Southeastern U.S." October 1, 2021. Panhandle Ag e-News UF/IFAS Extension. Accessed September 3, 2022. https://nwdistrict.ifas.ufl.edu/phag/2021/10/01/chagas-disease-a-health-risk-for-rural-people-in-the-southeastern-u-s/
World Health Organization (WHO) Expert Committee on the Control of Chagas Disease (2000: Brasilia, Brazil) & World Health Organization. 2002. Control of Chagas Disease: Second Report of the WHO Expert Committee. https://apps.who.int/iris/handle/10665/42443
World Health Organization (WHO). 2022. "Chagas Disease (Also Known as American Trypanosomiasis)." April 13, 2022. Accessed December 8, 2022. https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis).
World Health Organization (WHO). 2015. "Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates." Weekly Epidemiological Records 90:33–44. https://apps.who.int/iris/bitstream/handle/10665/242316/WER9006_33-44.PDF?sequence=1&isAllowed=y.
Zeledon, R., C. B. Beard, J. C. Pinto Dias, D. A. Leiby, P. L. Dorn, and J. R. Coura. 2012. "Triatomine Bug Vectors." In An Appraisal of the Status of Chagas Disease in the United States. Elsevier; Waltham, MA, USA: pp. 5–C1. https://doi.org/10.1016/B978-0-12-397268-2.00002-X