The Featured Creatures collection provides in-depth profiles of insects, nematodes, arachnids and other organisms relevant to Florida. These profiles are intended for the use of interested laypersons with some knowledge of biology as well as academic audiences.
This publication describes the biology, distribution, behavior, and impact of the parasitoid Leptomastix dactylopii Howard. This beneficial insect is known for providing significant reductions in mealybug populations in Florida and other locations. This document is also intended to provide knowledge about this parasitoid to a wide range of interested audiences including growers, Extension agents, researchers, students, laypersons, and other stakeholders.
Introduction
Mealybugs are a unique group of scale insects in the family Pseudococcidae and are reported as serious pests attacking citrus in Florida (Diepenbrock et al. 2022). Application of insecticides does not always have a favorable outcome in mealybug control programs due to their presence and feeding in the concealed locations, a waxy covering on their bodies, and resistance to insecticides (Diepenbrock et al. 2022). The solitary endoparasitoid Leptomastix dactylopii Howard (Hymenoptera: Encyrtidae) is a beneficial insect that targets approximately 20 species of mealybugs (Hemiptera: Pseudococcidae) (Noyes and Hayat 1994; Chong and Oetting 2007).
Distribution
There are about 35 described species of the genus Leptomastix worldwide (Anga and Noyes, 1999). Leptomastix dactylopii is thought to be native to South America and Africa (Bartlett 1978; Anga and Noyes, 1999) but later spread around the world (Figure 1). It has been frequently used as a biocontrol agent against mealybugs in the Middle East, Europe, United States, Pakistan, India, and Australia (Smith et al. 1988; Noyes and Hayat 1994; Cocco et al. 2021; Muştu et al. 2022).
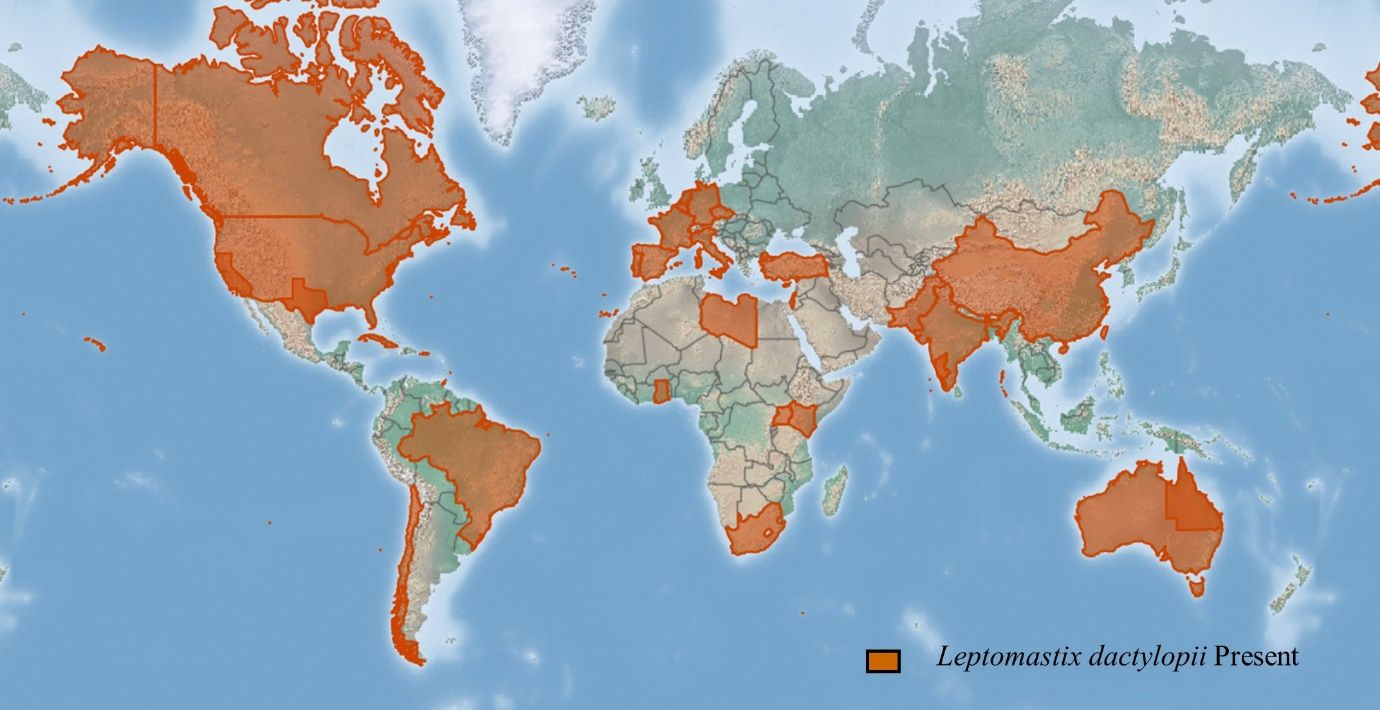
Credit: https://www.cabi.org/isc/datasheet/31522#toDistributionMaps
Description
Eggs
Females (Figure 2) lay only one egg per host individual (Yang and Sadof 1997; Marras et al. 2016) and prefer large size hosts including third and fourth instar nymphs (de Jong and van Alphen 1989; Muştu et al. 2022). Upon finding a suitable host, a female exhibits the behavior of examination, probing and oviposition (Chong and Oetting 2007). Interestingly, Leptomastix dactylopii is capable of distinguishing between unparasitized and parasitized hosts (Marras et al. 2016). A female Leptomastix dactylopii can lay up to 10 eggs a day (de Jong and van Alphen 1989) and about 51-66 eggs during her life span (Van Baaren and Nénon 1994; Yang and Sadof 1997). They fail to lay eggs in cold conditions and reproduce remarkably high at 26-30°C (78.8 - 86°F) (Tingle and Copland 1989; Battaglia and Tranfaglia 1994). There is a coating of spherulae containing a minute protein on the egg of Leptoamstix dactylopii that is believed to prevent its encapsulation by the host (Blumberg and Van Driesche 2001).

Credit: https://www.biocontrol.ch/fr_bc/leptomastix-dactylopii
Larvae
The mealybugs parasitized with Leptomastix dactylopii immediately stop feeding and never lay eggs (Zinna 1960). There are four larval instars of Leptomastix dactylopii and all feed and develop inside the host mealybug (Zinna 1960). The number of larval segments varies from 11 to 13, the last four segments are not clearly distinguished in the younger instars (Zinna 1960; Anga and Noyes 1999). Parasitized mealybugs turn brown in color and their bodies become bloated (Figure 3 top row). Muştu et al. (2022) reported that Leptomastix dactylopii female and male developmental time (egg to adult) is 16 and 15 days, respectively, when developing in third instar nymphs and 17 and 16 days, respectively, when developing in females of Planococcus ficus. Battaglia et al. (1996) reported a developmental duration for female and male Leptomastix dactylopii at 18 and 16 days, respectively.

Credit: Salman Al-Shami UF/IFAS
Pupae
The larvae develop into pupae inside the mealybug. The color of the mealybug with Leptomastix dactylopii pupating inside is brown to dark brown (Figure 3 bottom row).
Adults
Leptomastix dactylopii adults emerge by chewing an exit hole in the mummified mealybug (Figure 4 top row). The wasp completes its life cycle in the host within approximately 3-4 weeks depending on the temperature and size of mealybug (Muştu et al. 2022). At 25ºC (77°F), Leptomastix dactylopii can complete a generation in 16-18 days (Battaglia et al. 1996). According to Muştu et al. (2022), the total life span of parasitoid females and males in Plancoccous citri or Plancoccus ficus averaged 41 and 38 days, respectively. The adult is small {2-3 mm (~1/8 in)}, yellowish brown, and distinguishable by its long antennae which are darker in color and almost same length as the body (Anga and Noyes 1999). Females have bended antennae (Figure 4 bottom left), while males do not (Figure 4 bottom right). The adult can live for 2-3 weeks feeding on honeydew secreted by the mealybugs, nectar, and pollen.

Credit: Salman Al-Shami UF/IFAS
Hosts
Leptomastix dactylopii is an endoparasite of more than 20 mealybug species (Hemiptera: Pseudococcidae) including Ferrisia virgata (Cockerell), Phenacoccus madeirensis Green, Phenacoccus solani Ferris, Planococcus citri (Risso), Pseudococcus longispinus (Targioni-Tozzetti), Planococcus ficus and Pseudococcus viburni (Signoret) (Noyes and Hayat 1994; Chong and Oetting 2007). Several studies reported citrus mealybug Planococcus citri, vine mealybug Pseudococcus ficus, longtailed mealybug Pseudococcus longispinus and obscure mealybug Pseudococcus viburni as preferred hosts for this wasp (Mani et al. 2004; Chong and Oetting 2007; Mahfoudhi and Dhouibi 2009; Marras et al. 2016).
Biology and Ecology
Leptomastix species are primary, solitary endoparasitoids of mealybugs (Zinna 1959). Leptoamstix dactylopii progeny are dominated by females (Su and Li 1993). Survival is affected by several factors including food availability and temperature. This wasp is highly susceptible to low temperatures and lacks the ability for long overwintering (Franco et al. 2004; Mahfoudhi and Dhouibi 2009). According to Zinna (1960), the females of this parasitoid failed to lay eggs at temperatures lower than 18ºC (64.4°F). At an average temperature of 27ºC (80.6°F), females oviposited within a few hours after emergence (Lloyd 1958) and lived for a long time (Tingle and Copland 1989). Leptoamstix dactylopii is reported to target several species of mealybugs and shows a preference for some of the species known as significant agricultural pests such as citrus mealybug Planococcus citri, vine mealybug Planococcus ficus, longtailed mealybug Pseudococcus longispinus and obscure mealybug Pseudococcus viburni (Chong and Oetting 2007; Mahfoudhi and Dhouibi 2009).
Biological Control Agent Importance
Leptomastix dactypolii has been used to control the citrus mealybug Plancococcus citri and vine mealybug Pseudococcus ficus populations in fields and greenhouses (Doutt 1952; Krishnamoorthy and Singh 1987; Smith et al. 1988; Mani et al. 2011). In California, Leptomastix dactylopii has been used effectively to suppress vine mealybug Plancococcus ficus populations (Daane et al., 2008, 2012) and has been frequently found in vineyards of South Africa and Tunisia (Walton and Pringle 2004; Mahfoudhi and Dhouibi 2009). Krambias and Kontzonis (1980) reported a parasitism rate of 16% on citrus mealybug by Leptomastix dactypolii in Cyprus. In India, establishment of this parasitoid in two orchards resulted in successful control of citrus mealybug in 3-4 months (Krishnamoorthy and Singh 1987). Leptomastix dactypolii was also responsible for the significant reduction of citrus mealybug populations in Citrus Under Protective Screen (CUPS) in the Southeast of Florida (Al-Shami and Qureshi, unpublished). It is available commercially and can be an ideal biological control agent against citrus and vine mealybugs. This will help growers to control these mealybugs attacking crops in small nurseries and greenhouses or even at a larger scale in orchards. There is no information on the efficacy of Leptomastix dactypolii against the newly spreading lebbeck mealybug, Nipaecoccus viridis in citrus crops in Florida. Future investigation on the suitability of Leptomastix dactylopii to control the lebbeck mealybug will make a worthwhile contribution to current citrus IPM programs.
Selected references
Anga JM, Noyes JS. 1999. “A revision of the African and Malagasy species of the genus Leptomastix (Hymenoptera, Encyrtidae), parasitoids of mealybugs (Homoptera: Pseudococcidae).” Bulletin of The Natural History Museum (Entomology Series) 68: 93-128.
Battaglia D, Tranfaglia A, Franco JC, Carvalho CJ. 1996. “Leptomastix dactylopii Howard (Hymenoptera, Encyrtidae) fecundity and innate capacity for increase under laboratory controlled conditions.” Bollettino del Laboratorio di Entomologia Agraria 'Filippo Silvestri', Portici 18: 1-150.
BR. 1978. “Pseudococcidae.” In: CP(ed.), “Introduced parasites and predators of arthropod pests and weeds: A world review.” Agriculture handbook no. 480 (pp. 137–170). USDA Washington, DC.
Blumberg D, Van Driesche R.G. 2001. “Encapsulation rates of three encyrtid parasitoids by three mealybug species (Homoptera: Pseudococcidae) found commonly as pests in commercial greenhouses.” Biological Control 22: 191–199. https://doi.org/10.1006/bcon.2001.0966
JH, RD. 2007. “Specificity of Anagyrus sp. nov. nr. sinope and Leptomastix dactylopii for six mealybug species.” BioControl 52: 289–308. https://doi.org/10.1007/s10526-006-9025-5
Journal of Pest Sciencehttps://doi.org/10.1007/s10340-020-01305-8
Daane KM, Cooper ML, Triapitsyn SV, Walton VM, Yokota GY, Haviland DR, Bentley WJ, Godfrey KE, Wunderlich LR. 2008. “Vineyard managers and researchers seek sustainable solutions for mealybugs, a changing pest complex.” California Agriculture 62 (4): 167-176. https://doi.org/10.3733/ca.v062n04p167
Daane KM, Almeida RPP, Bell VA, Walker JTS, Botton M, Fallahzadeh M, Mani M, Miano JL, Sforza R, Walton VM, Zaviezo T. 2012. “Biology and management of mealybugs in vineyards.” In: Bostanian NJ, Charles V, Isaacs R. (eds.), “Arthropod Management in Vineyards: Pests, Approaches, and Future Directions.” Springer, New York, pp. 271-307. https://doi.org/10.1007/978-94-007-4032-7_12
de Jong P, and van Alphen JJM. 1989. “Host size selection and sex allocation in Leptoma.stix dactylopii, a parasitoid of Planococcus citri.” Entomologia Experimentalis et Applicata 50: 161-169. https://doi.org/10.1111/j.1570-7458.1989.tb02385.x
Diepenbrock LM, Stelinski L, Martini X, Qureshi J. 2022. “2022–2023 Florida Citrus Production Guide: Soft-Bodied Insects Attacking Foliage and Fruit: CPG Ch. 26, CG004/ENY-604, Rev. 3/2022”. EDIS 2022 (CPG). https://doi.org/10.32473/edis-cg004-2022
Doutt RL. 1952. “Biological control of Planococcus citri on commercial greenhouse Stephanotis.” Journal of Economic Entomology 45(2): 343-344. https://doi.org/10.1093/jee/45.2.343
Franco JC, Suma P, da Silva EB, Blumberg D, Mendel Z. 2004. “Management strategies of mealybug pests of citrus in Mediterranean countries.” Phytoparasitica 32: 507-522. https://doi.org/10.1007/BF02980445
Krambias A, Kontzonis A. 1980. “Establishment of Leptomastix dactylopii How. in Cyprus.” Fruits 35: 783-785.
Krishnamoorthy A, Singh SP. 1987. “Biological Control of Citrus Mealybug, Planococcus citri with an introduced parasite, Leptomastix dactylopii in India.” Entomophaga 32: 143-148. https://doi.org/10.1007/BF02373124
Lloyd DC. 1958. “Studies of Parasite Oviposition Behaviour.: II. Leptomastix dactylopii Howard (Hymenoptera, Encyrtidae).” The Canadian Entomologist (8):450-61. https://doi.org/10.4039/Ent90450-8
Mahfoudhi N, Dhouibi MH. 2009. “Survey of mealybugs (Hemiptera: Pseudococcidae) and their natural enemies in Tunisian vineyards.” African Entomology 17: 154-160. https://doi.org/10.4001/003.017.0205
Mani M. 1994. “Effectiveness of the exotic encyrtid parasitoid, Leptomastix dactylopii How. in the control of mealybug, Planococcus citri (Risso) in guava orchards.” Journal of Entomological Research 18: 351-355.
Marras PM, Cocco A, Muscas E, Lentini A. 2016. “Laboratory evaluation of the suitability of vine mealybug, Planococcus ficus, as a host for Leptomastix dactylopii.” Biological Control 95: 57–65. https://doi.org/10.1016/j.biocontrol.2016.01.007
Muştu M, Derya FN, Tarhanacı B. 2022. “Host stage preference and demographic parameters of Leptomastix dactylopii (Hymenoptera: Encyrtidae) on vine mealybug Planococcus ficus (Hemiptera: Pseudococcidae).” Egyptian Journal of Biological Pest Control 32:75. https://doi.org/10.1186/s41938-022-00572-0
Noyes JS, Hayat M. 1994. “Oriental Mealybug Parasitoids of the Anagyrini (Hymenoptera: Encyrtidae).” CAB International, Wallingford, Oxon, UK.
Smith D, Papacek DF, Murray DAH. 1988. “The use of Leptomastix dactylopii Howard (Hymenoptera: Encyrtidae) to control Planococcus citri (Risso) (Hemiptera: Pseudococcidae) in Queensland citrus orchards.” Queensland Journal of Agricultural and Animal Sciences 45: 157-164.
Su T, Li C. 1993. “Factors affecting the sex ratio of Leptomastix dactylopii Howard, a parasitoid of Planococcus citri (Risso).” Chinese Journal of Entomology 13(4): 319-329.
Tingle CC, Copland MJ. 1989. “Progeny production and adult longevity of the mealybug parasitoids Anagyrus pseudococci, Leptomastix dactylopii, and Leptomastidea abnormis [Hym.: Encyrtidae] in relation to temperature.” Entomophaga 34:111-20. https://doi.org/10.1007/BF02372594
Van Baaren J, Nénon JP. 1994. “Factors involved in host discrimination by Epidinocarsis lopezi and Leptomastix dactylopii (Hymenoptera: Encyrtidae).” Journal of Applied Entomology 118: 76–83. https://doi.org/10.1111/j.1439-0418.1994.tb00781.x
VM, KL. 2004. “A survey of mealybugs and associated natural enemies in vineyards in the Western Cape Province, South Africa.” South African Journal of Enology and Viticulture 25: 23-25. https://doi.org/10.21548/25-1-2134
Yang J, Sadof CS. 1997. “Variation in the life history of the citrus mealybug parasitoid Leptomastix dactylopii (Hymenoptera: Encyrtidae) on three varieties of Coleus blumei.” Environmental Entomology 26: 978-982. https://doi.org/10.1093/ee/26.4.978
Zinna G. 1959. “Ricerche sugli insetti entomofagi I. Specializzazione entomoparassitica negli Encyrtidae. Studio morfologico, etologico e fisiologico del Leptomastix dactylopii Howard.” Bollettino del Laboratorio di Entomologia Agraria 'Filippo Silvestri', Portici 18:1–148.
Zinna G. 1960. Esperimenti di lotta biologica controil cotonello degli agrumi (Pseudococcus citri (Risso)) nell'Isola di Procida mediante l'impiego di due parassiti esotica, Pauridia peregrina Timb. e Leptomastix dactylopii How. Bollettino del Laboratorio di Entomologia Agraria 'Filippo Silvestri', Portici 28: 257-284.