This publication aims to provide information on differentiating damage caused by chilli thrips, spider mites, foliar nematodes, and phytotoxicity due to herbicide application on strawberry plants. The symptomology of these pests and the signs of herbicide damage can appear similar on plants, and producers need to observe carefully to determine the actual cause of damage. Correctly identifying the cause behind each damage symptom is essential to solving the problem. The intended audience of this article is strawberry growers, crop scouts, Extension agents, and industry personnel working on improved production and management of strawberries.
Chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), is an invasive pest that can feed on many host plants, including strawberries. Chilli thrips prefer to feed on tender plant tissue, but can feed on all the above-ground plant parts. Chilli thrips feed on the cell content of the leaves using their piercing-sucking mouth parts, resulting in feeding scars and tissue necrosis (Kumar et al. 2013). The feeding damage on strawberry leaves can be observed as red or bronze streaks starting from the midvein (Figure 1 A–C). Heavy infestation leads to distorted leaves, which curl upward. It is important not to confuse this with auxinic herbicide damage, which causes petiole twisting and upward curling of the leaves.

Credit: Lovely Adhikary, UF/IFAS GCREC
Another important strawberry pest, twospotted spider mite (TSSM), Tetranychus urticae Koch (Arachnida: Acari), also causes damage to the strawberry leaf. The TSSM has piercing-sucking mouthparts that feed on the plant sap by penetrating the tissue (Fasulo 2003). These mites damage the mesophyll, the middle layer of a leaf that contains chloroplasts which is the main region of photosynthesis. As a result of the feeding of TSSM, mesophyll tissue collapses, and chlorotic spots appear on the damaged leaves. Twospotted spider mites are ravenous feeders that can feed on 18–22 cells per minute (Fasulo 2003). Extended feeding by TSSM causes “stippling” symptoms on the leaf (Figure 2 A–B). The obvious symptom of spider mite infestation is the presence of webs (Figure 2C).

Credit: Lovely Adhikary, UF/IFAS GCREC
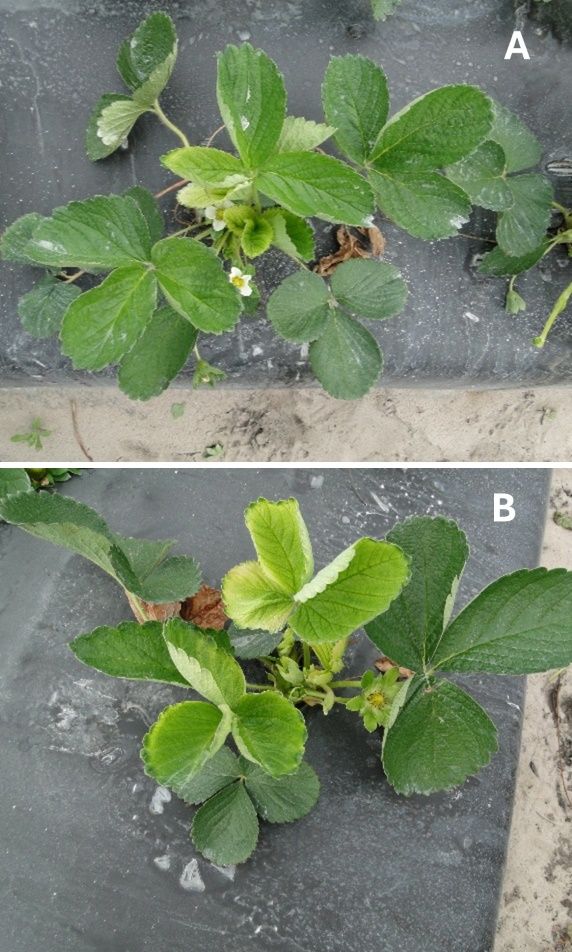
Foliar or bud nematodes (Aphelenchoides spp.) can produce significant above-ground damage to strawberries all over the world. Three species are known to cause damage to strawberries: Aphelenchoides besseyi, A. fragariae, and A. ritzemabosi. In Florida, the main species is Aphelenchoides besseyi. This nematode has been known to cause dwarfing or crimp symptoms on strawberries in Florida since the early 1900s (Desaeger and Noling 2017). In strawberries, the nematodes move outside the plant tissue (mostly in the protected spaces at the bases of young leaves) and feed by inserting their hypodermic stylet into leaf tissue. As a result of their feeding, the leaves of infected plants become distorted, crinkled, and darker green. Young plants are often dwarfed. Reddish spots can be visible on the leaf edge, which eventually dries up and turns brown. Severely infected plants may remain stunted or, in some cases produce excessive vegetative growth. They frequently fail to produce flowers, and the few fruits they manage to produce are almost always unmarketable, as the infestation results in deformed or cat-faced fruits (Desaeger and Noling 2017; Desaeger 2022). High humidity aids in the spread of these nematodes because they move easily through thin layers of water (Desaeger and Noling 2017).

Credit: Johan Desaeger, UF/IFAS GCREC
Pre-emergence herbicides such as flumioxazin (Chateau), S-metolachlor (Dual Magnum), and sulfentrazone (Spartan 4F), as well as post-emergence herbicides such as glyphosate (Roundup), carfentrazone (Aim), and gramoxone (Paraquat), are commonly used in strawberry cultivation and may cause phytotoxicity (Figure 4, Figure 5. A–B, Figure 6. A–B).

Credit: Sriyanka Lahiri, UF/IFAS GCREC
Credit: Nathan Boyd, UF/IFAS GCREC
Flumioxazin inhibits chlorophyll synthesis and disrupts cell membranes (Hutchinson 2007). This herbicide is commonly applied on the soil surface under the plastic mulch; phytotoxicity can occur when it is applied in poorly drained soils or at a very high rate (Boyd and Yu 2018). Typical symptoms include purple-colored veins (Figure 4) and speckling on foliage when herbicides are applied post-emergence.
S-metolachlor is also applied as a pre-emergence herbicide. The phytotoxicity symptoms include discoloration and deformation of leaves, with the petiole curling upward. As this herbicide can be absorbed by both the plant shoot and roots, and metabolized before it causes any harm to the leaves, the pre-emergence application method can reduce the chances of injury. Application through a drip tube is safer than bed top application in strawberries (Boyd and Reed 2016).
Sulfentrazone injury on plants includes chlorotic spots, crinkled leaves, and stunted growth (Taylor-Lovell et al. 2001). The phytotoxicity of sulfentrazone depends on soil organic matter content and soil pH. Injury levels increased with increased soil pH (Szmigielski et al. 2009).
Non-adsorbed glyphosate can potentially cause phytotoxicity on strawberry plant tissue (Kanissery et al. 2019) when exposed accidentally or via drift. Injury symptoms include yellowing or chlorosis on the young leaves and growing plant parts (Rodriguez Salamanca et al. 2023). If exposed to accidental application, yellowing is followed by necrosis, which affects every part of the plant.

Credit: Emily R. Witt, UF/IFAS GCREC
The post-emergence herbicide, carfentrazone blocks chlorophyll synthesis. Phytotoxicity symptoms of this herbicide include necrotic lesions on the leaves (Rodriguez Salamanca et al. 2023). Plants may survive the initial injury caused by accidental exposure to carfentrazone (Allen 2015).
The other post-emergence herbicide paraquat, generates reactive oxygen species, leading to damage of cell membranes, which results in cell death (Li et al. 2013). Phytotoxicity may occur from applying paraquat because of the production of free radicals (Putnam and Ries 1968). Symptoms include necrotic spots on the leaves and water-soaked areas that later become yellow or brown spots (Rodriguez Salamanca et al. 2023).
Sometimes, herbicide phytotoxicity produces symptoms similar to those of arthropod or nematode pests on the strawberry leaves. For example, the phytotoxicity of Chateau results in dark purple stripes on the leaf along the vein (Fig. 4), which is almost identical to chilli thrips damage (Fig. 1C). Also, Chateau drift produces chlorotic spots, which can appear similar to spider mite damage (Fig. 2A–B). Thickened dark leaves and a stunted canopy on strawberry plants caused by foliar nematodes (Fig. 3A–B) can also be confused with heavy chilli thrips infestation. However, there are some definite symptoms of each of these issues described in Table 1.
Table 1. Comparison of symptoms from chilli thrips, spider mites, foliar nematode damage, and herbicide phytotoxicity on strawberry plants.
Conclusion
Primary assessment of plausible causes of visible plant damage can be done to rule out possibilities, as outlined in Table 1. The first step toward an effective management plan is to consult agricultural Extension services or send samples to a plant diagnostic center to identify the correct reason for the damage symptoms on the leaves. Once the cause is determined, consult with Extension personnel to determine the best course of action.
References
Allen, J. 2015. Final Project Report 2015. Strawberry: Investigating Rates and Application Timing of Carfentrazone-Ethyl (Shark). https://projectbluearchive.blob.core.windows.net/media/Default/Research%20Papers/Horticulture/SF%20151_Report_Final_2015.pdf.
Boyd, N. S., and T. Reed. 2016. “Strawberry Tolerance to Bed-Top and Drip-Applied Preemergence Herbicides.” Weed Technology 30:492–498. https://doi.org/10.1614/WT-D-15-00149.1
Boyd, N. S., and J. Yu. 2018 FSREF Research Report 2017–18. UF/IFAS Extension. https://member.floridastrawberry.org/wp-content/uploads/2018/07/FSREF-2017-18-Boyd-Chateau-Research-Report.pdf
Desaeger. J. 2022. “Recognizing and Managing Nematode Damage in Strawberries.” Southern Region Small Fruit Consortium. College of Agricultural and Environmental Sciences, University of Georgia. https://smallfruits.org/2022/01/nematode-damage-in-strawberries/
Desaeger, J., and J. W. Noling. 2017. “Foliar or Bud Nematodes in Florida Strawberries: ENY-068/IN1184, 11/2017.” EDIS 2017 (6) Gainesville, FL. https://doi.org/10.32473/edis-in1184-2017
Fasulo, Thomas R., and Harold A. Denmark. 2003. “Twospotted Spider Mite, Tetranychus Urticae Koch (Arachnida: Acari: Tetranychidae): EENY150 IN307, 8 2000.” EDIS 2003 (15). Gainesville, FL. https://doi.org/10.32473/edis-in307-2000
Hutchinson, P. J. 2007. Chateau Herbicide for Use in Potatoes. College of Agriculture and Life Sciences, University of Idaho. Accessed on January 26, 2024.
Kanissery, R., B. Gairhe, D. Kadyampakeni, O. Batuman, and F. Alferez. 2019. “Glyphosate: Its Environmental Persistence and Impact on Crop Health and Nutrition.” Plants 8:499. https://doi.org/10.3390/plants8110499
Kumar, V., G. Kakkar, C. L. McKenzie, D. R. Seal, and L. S. Osborne. 2013. “An Overview of Chilli Thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) Biology, Distribution, and Management.” Weed and Pest Control—Conventional and New Challenges 53–77. https://doi.org/10.5772/55045
Li, J., J. Mu, J. Bai, et al. 2013. “Paraquat Resistant1, a Golgi-Localized Putative Transporter Protein, Is Involved in Intracellular Transport of Paraquat.” Plant Physiology 162:470–483. https://doi.org/10.1104/pp.113.213892
Putnam, A. R., and S. K. Ries. 1968. “Factors Influencing the Phytotoxicity and Movement of Paraquat in Quackgrass.” Weed Science 16:80–83. https://doi.org/10.1017/S0043174500030605
Rodriguez Salamanca, L. M., A. Steil, B. Hartzler, and B. Baker. 2023. “Horticulture and Home Pest News: Herbicide Injury to Garden Plants.” Iowa State University, Extension and Outreach. https://yardandgarden.extension.iastate.edu/encyclopedia/herbicide-injury-garden-plants
Szmigielski, A. M., J. J. Schoenau, E. N. Johnson, F. A. Holm, K. L. Sapsford, and J. Liu. 2009. “Development of a Laboratory Bioassay and Effect of Soil Properties on Sulfentrazone Phytotoxicity in Soil.” Weed Technology 23:486–491. https://doi.org/10.1614/WT-08-122.1
Taylor-Lovell, S., L. M. Wax, and R. Nelson. 2001. “Phytotoxic Response and Yield of Soybean (Glycine max) Varieties Treated with Sulfentrazone or Flumioxazin.” Weed Technology 15:95–102. https://doi.org/10.1614/0890-037X(2001)015[0095:PRAYOS]2.0.CO;2